XIST knockdown suppresses vascular smooth muscle cell proliferation and induces apoptosis by regulating miR-1264/WNT5A/β-catenin signaling in aneurysm
- PMID: 33501488
- PMCID: PMC7960886
- DOI: 10.1042/BSR20201810
XIST knockdown suppresses vascular smooth muscle cell proliferation and induces apoptosis by regulating miR-1264/WNT5A/β-catenin signaling in aneurysm
Abstract
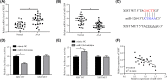
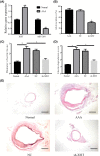
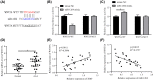
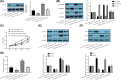
Long non-coding RNAs (lncRNAs) have been ascertained as vital modulators in abdominal aortic aneurysm (AAA) development. In this research, the function and molecular mechanisms of the lncRNA X-inactive specific transcript (XIST) in the evolution of vascular smooth muscle cells (VSMCs) were assessed. Results showed that XIST expression was increased but miR-1264 expression level was reduced in the serum of AAA patients. XIST depletion impeded human aorta VSMCs (HA-VSMCs') ability to proliferate and stimulate apoptosis, while repressing miR-1264 expression through an unmediated interaction. Additionally, the influence of XIST knockdown on apoptosis and proliferation could be rescued by an miR-1264 inhibitor. Subsequent molecular investigations indicated that WNT5A was miR-1264's target, and XIST functioned as a competing endogenous RNA (ceRNA) of miR-1264 to raise WNT5A expression. Further, an miR-1264 inhibitor stimulated the proliferation and suppressed the apoptosis of HA-VSMCs through the activation of WNT/β-catenin signaling. Taken together, XIST impeded the apoptosis and stimulated the proliferation of HA-VSMCs via the WNT/β-catenin signaling pathway through miR-1264, demonstrating XIST's underlying role in AAA.
Keywords: abdominal aortic aneurysm; lncRNA; miR-1264; proliferation.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

