Ivabradine use in pregnant women-treatment indications and pregnancy outcome: an evaluation of the German Embryotox database
- PMID: 33501507
- PMCID: PMC8184534
- DOI: 10.1007/s00228-020-03066-w
Ivabradine use in pregnant women-treatment indications and pregnancy outcome: an evaluation of the German Embryotox database
Abstract
Purpose: Ivabradine has been approved for the treatment of chronic heart failure and chronic stable angina pectoris in Europe. Based on adverse outcomes of reproductive animal studies and the lack of human data, ivabradine is considered contraindicated during pregnancy. The aim of this observational study is to analyse ivabradine use before and during pregnancy.
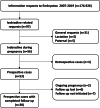
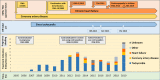
Methods: We evaluated all ivabradine-related requests to the German Embryotox Institute from 2007 to 2019. Exposed pregnancies were analysed as to their outcome.
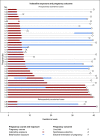
Results: Off-label use for supraventricular tachycardia was frequent in women of childbearing age. Of 38 prospectively ascertained pregnancies with ivabradine exposure and completed follow-up, 32 resulted in live births, 3 in spontaneous abortions, and 3 were electively terminated. One neonate presented with major birth defects (atrial septal defect and cleft palate). In 33/38 patients, ivabradine was discontinued after confirmation of pregnancy without cardiac deterioration and 5/38 women continued ivabradine throughout pregnancy. In addition, there were 3 retrospectively reported pregnancies including one major birth defect (tracheal atresia).
Conclusion: This case series represents the largest cohort of ivabradine-exposed pregnancies, published so far. According to our findings, ivabradine appears not to be a major teratogen. However, established drugs of choice with strong evidence of low risk for the unborn should be preferred in women planning pregnancy. After inadvertent exposure during pregnancy or lack of treatment alternatives, fetal ultrasound for structural anomalies and growth restriction is recommended. In addition, close monitoring is necessary in pregnant women with supraventricular arrhythmias or cardiac disease.
Keywords: Drug safety; Ivabradine; Pregnancy; Reproductive age; Tachycardia.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Pregnancy outcome after exposure to the novel oral anticoagulant rivaroxaban in women at suspected risk for thromboembolic events: a case series from the German Embryotox Pharmacovigilance Centre.Clin Res Cardiol. 2016 Feb;105(2):117-26. doi: 10.1007/s00392-015-0893-5. Epub 2015 Jul 21. Clin Res Cardiol. 2016. PMID: 26195125
-
Pregnancy outcome after first trimester exposure to bisoprolol: an observational cohort study.J Hypertens. 2018 Oct;36(10):2109-2117. doi: 10.1097/HJH.0000000000001818. J Hypertens. 2018. PMID: 29985206
-
Pregnancy outcome after first-trimester exposure to fosfomycin for the treatment of urinary tract infection: an observational cohort study.Infection. 2020 Feb;48(1):57-64. doi: 10.1007/s15010-019-01342-1. Epub 2019 Jul 13. Infection. 2020. PMID: 31302868
-
Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes.Drug Saf. 2007;30(3):247-64. doi: 10.2165/00002018-200730030-00006. Drug Saf. 2007. PMID: 17343431 Review.
-
Ivabradine and endothelium: an update.Ther Adv Cardiovasc Dis. 2020 Jan-Dec;14:1753944720934937. doi: 10.1177/1753944720934937. Ther Adv Cardiovasc Dis. 2020. PMID: 32611276 Free PMC article. Review.
Cited by
-
What Do We Know about Peripartum Cardiomyopathy? Yesterday, Today, Tomorrow.Int J Mol Sci. 2024 Sep 30;25(19):10559. doi: 10.3390/ijms251910559. Int J Mol Sci. 2024. PMID: 39408885 Free PMC article. Review.
-
Ivabradine-sensitive incessant atrial tachycardia during pregnancy: a case report.Eur Heart J Case Rep. 2021 Sep 28;5(10):ytab367. doi: 10.1093/ehjcr/ytab367. eCollection 2021 Oct. Eur Heart J Case Rep. 2021. PMID: 34632265 Free PMC article.
References
-
- EMA, Committee for Medicinal Products for Human Use (CHMP) (2011) Summary of opinion: Procoralan. Ivabradine. Accessed 8.9.2020 from https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-posi...
-
- FDA (2015) Pharmacology review(s). Accessed 8.9.2020 from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206143Orig1s000P...
-
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, GMC R, Seeland U, Simoncini T, Swan L, Warnes CA, Group ESCSD 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

