Endogenous animal models of intracranial aneurysm development: a review
- PMID: 33501561
- PMCID: PMC8310898
- DOI: 10.1007/s10143-021-01481-w
Endogenous animal models of intracranial aneurysm development: a review
Abstract
The pathogenesis and natural history of intracranial aneurysm (IA) remains poorly understood. To this end, animal models with induced cerebral vessel lesions mimicking human aneurysms have provided the ability to greatly expand our understanding. In this review, we comprehensively searched the published literature to identify studies that endogenously induced IA formation in animals. Studies that constructed aneurysms (i.e., by surgically creating a sac) were excluded. From the eligible studies, we reported information including the animal species, method for aneurysm induction, aneurysm definitions, evaluation methods, aneurysm characteristics, formation rate, rupture rate, and time course. Between 1960 and 2019, 174 articles reported endogenous animal models of IA. The majority used flow modification, hypertension, and vessel wall weakening (i.e., elastase treatment) to induce IAs, primarily in rats and mice. Most studies utilized subjective or qualitative descriptions to define experimental aneurysms and histology to study them. In general, experimental IAs resembled the pathobiology of the human disease in terms of internal elastic lamina loss, medial layer degradation, and inflammatory cell infiltration. After the early 2000s, many endogenous animal models of IA began to incorporate state-of-the-art technology, such as gene expression profiling and 9.4-T magnetic resonance imaging (MRI) in vivo imaging, to quantitatively analyze the biological mechanisms of IA. Future studies aimed at longitudinally assessing IA pathobiology in models that incorporate aneurysm growth will likely have the largest impact on our understanding of the disease. We believe this will be aided by high-resolution, small animal, survival imaging, in situ live-cell imaging, and next-generation omics technology.
Keywords: Animal model; Cerebral aneurysm; Intracranial aneurysm; Natural history; Review; Vascular disease.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH, DE part of Springer Nature.
Conflict of interest statement
Conflicts of Interest/Competing Interests
VMT – Principal investigator: National Science Foundation Award No. 1746694, NIH NINDS award R43 NS115314–0, Clinical and Translational Science Institute grant. Co-founder: Neurovascular Diagnostics, Inc.
HR-O – None.
SSV – None.
KEP – None.
MW – None.
MM – None.
NL – None.
AHS – Financial Interest/Investor/Stock Options/Ownership: Amnis Therapeutics, Apama Medical, BlinkTBI, Inc, Buffalo Technology Partners, Inc., Cardinal Health, Cerebrotech Medical Systems, Inc, Claret Medical, Cognition Medical, Endostream Medical, Ltd, Imperative Care, International Medical Distribution Partners, Rebound Therapeutics Corp., Silk Road Medical, StimMed, Synchron, Three Rivers Medical, Inc., Viseon Spine, Inc.
Consultant/Advisory Board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA, Inc., Cerebrotech Medical Systems, Inc., Cerenovus, Claret Medical, Corindus, Inc., Endostream Medical, Ltd, Guidepoint Global Consulting, Imperative Care, Integra, Medtronic, Micro-Vention, Northwest University—DSMB Chair for HEAT Trial, Penumbra, Rapid Medical, Rebound Therapeutics Corp., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, W.L. Gore & Associates. National PI/Steering Committees: Cerenovus LARGE Trial and ARISE II Trial, Medtronic SWIFT PRIME and SWIFT DIRECT Trials, MicroVention FRED Trial & CONFIDENCE Study, MUSC POSITIVE Trial, Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial. Principal investigator: Cummings Foundation grant.
HM – Principal investigator NIH grant R01NS064592 and NIH grant R01NS091075. Co-founder: Neurovascular Diagnostics, Inc.
JK – None.
Figures
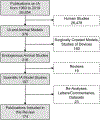





References
-
- Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10(7): 626–36. - PubMed
-
- Hackenberg KA, Hänggi D, Etminan N. Unruptured intracranial aneurysms: contemporary data and management. Stroke 2018; 49(9): 2268–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

