Saliva samples for detection of SARS-CoV-2 in mildly symptomatic and asymptomatic patients
- PMID: 33501645
- PMCID: PMC8013767
- DOI: 10.1002/jmv.26821
Saliva samples for detection of SARS-CoV-2 in mildly symptomatic and asymptomatic patients
Abstract
Background: The ongoing coronavirus disease 2019 (Covid-19) pandemic has been rapidly spreading throughout the world with confirmed case numbers already exceeding 75 million. Although nasopharyngeal swabs are the most commonly utilized samples for based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA detection, collecting these specimens requires healthcare workers and necessitates the use of personal protective equipment as it presents a nosocomial transmission risk. We aimed to assess the diagnostic value of saliva samples in mildly symptomatic and asymptomatic patients with confirmed Covid-19.
Methods: We performed a cohort study to validate the use of saliva for SARS-CoV-2 detection in mildly symptomatic and asymptomatic patients with a confirmed diagnosis of Covid-19. Saliva samples of the patients were analyzed by reverse-transcriptase polymerase chain reaction (RT-PCR).
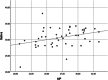
Results: In May 2020, 28 asymptomatic and 25 mildly symptomatic patients were enrolled in the study. The median age was 37 years (range 4-70). None of the patients had a fever on presentation. Among 53 patients with SARS-CoV-2 detected in the nasopharyngeal sample, the real-time RT-PCR was positive in the saliva specimens in 48 (90.56%) patients. The mean cycle threshold (CT) values for nasopharyngeal and saliva specimens (27.80 ± 3.44 and 30.64 ± 2.83, respectively) were significantly correlated between the two sample types (p = .016). The mean CT values of nasopharyngeal and saliva samples in mildly symptomatic and asymptomatic patients (27.18 ± 3.53 and 30.24 ± 3.29 vs. 28.36 ± 3.31 and 30.98 ± 2.39, respectively) were not significantly different (p = .236 and p = .733, respectively).
Conclusions: Saliva specimens can be considered as a reliable and less resource-intensive alternative to nasopharyngeal specimens for screening asymptomatic SARS-CoV-2 infections.
Keywords: Covid-19; RT-PCR; SARS-CoV-2; diagnosis; saliva.
© 2021 Wiley Periodicals LLC.
Figures
References
-
- World Health Organization . Coronavirus disease (COVID‐19) situation report. https://www.who.int/docs/default-source/coronaviruse/20200630-covid-19-s... (accessed December 20, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous