Suboptimal outcome for patients with biliary rhabdomyosarcoma treated on low-risk clinical trials: A report from the Children's Oncology Group
- PMID: 33501771
- PMCID: PMC8765674
- DOI: 10.1002/pbc.28914
Suboptimal outcome for patients with biliary rhabdomyosarcoma treated on low-risk clinical trials: A report from the Children's Oncology Group
Abstract
Background: Biliary rhabdomyosarcoma (RMS) is the most common biliary tumor in children. The biliary tract is classified as a favorable primary site. Therefore, patients with localized biliary RMS were included in two consecutive low-risk studies, D9602 and ARST0331, by the Children's Oncology Group (COG). The outcome for these patients treated with low-risk therapy has not been reported.
Procedure: Patients with biliary RMS enrolled on COG low-risk trials D9602 or ARST0331 were analyzed. All patients received systemic chemotherapy and those with Group II (microscopic residual) or Group III (macroscopic residual) disease received 36-50.4 Gy adjuvant radiotherapy (RT). Delayed primary excision (DPE) was allowed on both studies.
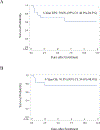
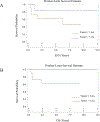
Results: Seventeen patients with biliary RMS were treated on D9602 (n = 7) or ARST0331 (n = 10). Median age was 3.5 years (range 1.7-10.3). Ten (59%) patients had tumors >5 cm and 14 (82%) had Group III disease. Fifteen (88%) patients received RT. The 5-year event-free survival (EFS) and overall survival (OS) were 70.6% (95% confidence interval [CI]: 46.9-94.3%) and 76.5% (95% CI: 54.6-98.4%), respectively. The majority of patients (80%) who received RT did not have disease recurrence while both patients who did not receive RT had local relapse. Five (36%) of 14 patients with Group III disease underwent DPE; two experienced a local relapse. In the nine patients without DPE, two developed local relapse.
Conclusions: Patients with localized biliary RMS treated on low-risk studies had suboptimal outcomes. These patients may benefit from therapy on intermediate-risk studies.
Keywords: biliary rhabdomyosarcoma; chemotherapy; radiotherapy; surgery.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest
The authors declare no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures
Comment in
-
Comment on: Patients with nonmetastatic embryonal rhabdomyosarcoma arising in the biliary tract should be treated on low-risk clinical trials.Pediatr Blood Cancer. 2022 Jul;69(7):e29554. doi: 10.1002/pbc.29554. Epub 2021 Dec 28. Pediatr Blood Cancer. 2022. PMID: 34962698 No abstract available.
References
-
- Wilks S, Moxon W: Lectures on Pathological Anatomy. 2nd ed. London, England, Longmans, Green, & Co, 1875, p. 462–467.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources