Voltage Imaging with a NIR-Absorbing Phosphine Oxide Rhodamine Voltage Reporter
- PMID: 33501825
- PMCID: PMC7986050
- DOI: 10.1021/jacs.0c11382
Voltage Imaging with a NIR-Absorbing Phosphine Oxide Rhodamine Voltage Reporter
Abstract
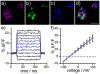
The development of fluorescent dyes that emit and absorb light at wavelengths greater than 700 nm and that respond to biochemical and biophysical events in living systems remains an outstanding challenge for noninvasive optical imaging. Here, we report the design, synthesis, and application of near-infrared (NIR)-absorbing and -emitting optical voltmeter based on a sulfonated, phosphine-oxide (po) rhodamine for voltage imaging in intact retinas. We find that po-rhodamine based voltage reporters, or poRhoVRs, display NIR excitation and emission profiles at greater than 700 nm, show a range of voltage sensitivities (13 to 43% ΔF/F per 100 mV in HEK cells), and can be combined with existing optical sensors, like Ca2+-sensitive fluorescent proteins (GCaMP), and actuators, like light-activated opsins ChannelRhodopsin-2 (ChR2). Simultaneous voltage and Ca2+ imaging reveals differences in activity dynamics in rat hippocampal neurons, and pairing poRhoVR with blue-light based ChR2 affords all-optical electrophysiology. In ex vivo retinas isolated from a mouse model of retinal degeneration, poRhoVR, together with GCaMP-based Ca2+ imaging and traditional multielectrode array (MEA) recording, can provide a comprehensive physiological activity profile of neuronal activity, revealing differences in voltage and Ca2+ dynamics within hyperactive networks of the mouse retina. Taken together, these experiments establish that poRhoVR will open new horizons in optical interrogation of cellular and neuronal physiology in intact systems.
Figures






References
-
- Power RM; Huisken J, A guide to light-sheet fluorescence microscopy for multiscale imaging. Nature Methods 2017, 14, 360. - PubMed
-
- Chung K; Wallace J; Kim S-Y; Kalyanasundaram S; Andalman AS; Davidson TJ; Mirzabekov JJ; Zalocusky KA; Mattis J; Denisin AK; Pak S; Bernstein H; Ramakrishnan C; Grosenick L; Gradinaru V; Deisseroth K, Structural and molecular interrogation of intact biological systems. Nature 2013, 497, 332. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

