Influenza Causes MLKL-Driven Cardiac Proteome Remodeling During Convalescence
- PMID: 33501852
- PMCID: PMC8313652
- DOI: 10.1161/CIRCRESAHA.120.318511
Influenza Causes MLKL-Driven Cardiac Proteome Remodeling During Convalescence
Abstract
Rationale: Patients with and without cardiovascular diseases have been shown to be at risk of influenza-mediated cardiac complications. Recent clinical reports support the notion of a direct link between laboratory-confirmed influenza virus infections and adverse cardiac events.
Objective: Define the molecular mechanisms underlying influenza virus-induced cardiac pathogenesis after resolution of pulmonary infection and the role of necroptosis in this process.
Methods and results: Hearts from wild-type and necroptosis-deficient (MLKL [mixed lineage kinase domain-like protein]-KO) mice were dissected 12 days after initial influenza A virus (IAV) infection when viral titers were undetectable in the lungs. Immunofluorescence microscopy and plaque assays showed presence of viable IAV particles in the myocardium without generation of interferon responses. Global proteome and phosphoproteome analyses using high-resolution accurate mass-based LC-MS/MS and label-free quantitation showed that the global proteome as well as the phosphoproteome profiles were significantly altered in IAV-infected mouse hearts in a strain-independent manner. Necroptosis-deficient mice had increased survival and reduced weight loss post-IAV infection, as well as increased antioxidant and mitochondrial function, indicating partial protection to IAV infection. These findings were confirmed in vitro by pretreatment of human and rat myocytes with antioxidants or necroptosis inhibitors, which blunted oxidative stress and mitochondrial damage after IAV infection.
Conclusions: This study provides the first evidence that the cardiac proteome and phosphoproteome are significantly altered post-pulmonary influenza infection. Moreover, viral particles can persist in the heart after lung clearance, altering mitochondrial function and promoting cell death without active replication and interferon responses. Finally, our findings show inhibition of necroptosis or prevention of mitochondrial damage as possible therapeutic interventions to reduce cardiac damage during influenza infections. Graphic Abstract: A graphic abstract is available for this article.
Keywords: cell death; heart; human influenza; mitochondria; necroptosis; oxidative stress; proteomics.
Figures

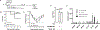

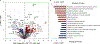
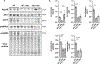


Similar articles
-
CYP1B1 knockout enhanced IFN-γ production is required but not sufficient for protection of cigarette smoke-exposed mice against lethal influenza virus infection.Front Immunol. 2025 Jul 4;16:1600025. doi: 10.3389/fimmu.2025.1600025. eCollection 2025. Front Immunol. 2025. PMID: 40688072 Free PMC article.
-
Dusp14-Mediated Dephosphorylation of MLKL Protects Against Cardiomyocyte Necroptosis in Hypothyroidism-Induced Heart Failure.Circulation. 2025 Jun 24;151(25):1797-1813. doi: 10.1161/CIRCULATIONAHA.125.074353. Epub 2025 May 13. Circulation. 2025. PMID: 40357546
-
PDGFRα-positive cell-derived TIMP-1 modulates adaptive immune responses to influenza A viral infection.Am J Physiol Lung Cell Mol Physiol. 2025 Jan 1;328(1):L60-L74. doi: 10.1152/ajplung.00104.2024. Epub 2024 Nov 25. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39585242 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Necroptosis Drives Major Adverse Cardiovascular Events During Severe COVID-19.Res Sq [Preprint]. 2023 Jan 21:rs.3.rs-2468706. doi: 10.21203/rs.3.rs-2468706/v1. Res Sq. 2023. Update in: Crit Care. 2023 Apr 20;27(1):155. doi: 10.1186/s13054-023-04423-8. PMID: 36711834 Free PMC article. Updated. Preprint.
-
Cardiomyocyte-specific NHE1 overexpression confers protection against myocardial infarction during hyperglycemia.Cardiovasc Diabetol. 2025 Apr 26;24(1):184. doi: 10.1186/s12933-025-02743-3. Cardiovasc Diabetol. 2025. PMID: 40287728 Free PMC article.
-
Kinetic Multi-omic Analysis of Responses to SARS-CoV-2 Infection in a Model of Severe COVID-19.J Virol. 2021 Sep 27;95(20):e0101021. doi: 10.1128/JVI.01010-21. Epub 2021 Jul 28. J Virol. 2021. PMID: 34319784 Free PMC article.
-
Need for standardization of Influenza A virus-induced cell death in vivo to improve consistency of inter-laboratory research findings.Cell Death Discov. 2024 May 22;10(1):247. doi: 10.1038/s41420-024-01981-w. Cell Death Discov. 2024. PMID: 38778049 Free PMC article. Review.
-
COVID-19 HEART unveiling as atrial fibrillation: pathophysiology, management and future directions for research.Egypt Heart J. 2023 Apr 30;75(1):36. doi: 10.1186/s43044-023-00359-0. Egypt Heart J. 2023. PMID: 37120772 Free PMC article. Review.
References
-
- Heron M Deaths: Leading causes for 2017. National vital statistics reports. 2019;68 - PubMed
-
- Paules C, Subbarao K. Influenza. Lancet. 2017;390:697–708 - PubMed
-
- Noda T [orthomyxoviruses]. Uirusu. 2012;62:219–228 - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the united states. JAMA. 2003;289:179–186 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

