Loss of the K+ channel Kv2.1 greatly reduces outward dark current and causes ionic dysregulation and degeneration in rod photoreceptors
- PMID: 33502442
- PMCID: PMC7845921
- DOI: 10.1085/jgp.202012687
Loss of the K+ channel Kv2.1 greatly reduces outward dark current and causes ionic dysregulation and degeneration in rod photoreceptors
Abstract
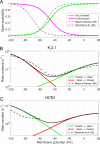
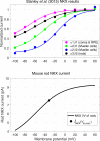
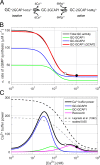
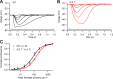
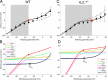
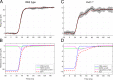
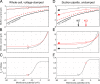
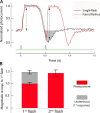
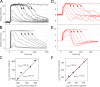
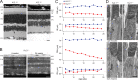
Vertebrate retinal photoreceptors signal light by suppressing a circulating "dark current" that maintains their relative depolarization in the dark. This dark current is composed of an inward current through CNG channels and NCKX transporters in the outer segment that is balanced by outward current exiting principally from the inner segment. It has been hypothesized that Kv2.1 channels carry a predominant fraction of the outward current in rods. We examined this hypothesis by comparing whole cell, suction electrode, and electroretinographic recordings from Kv2.1 knockout (Kv2.1-/-) and wild-type (WT) mouse rods. Single cell recordings revealed flash responses with unusual kinetics, and reduced dark currents that were quantitatively consistent with the measured depolarization of the membrane resting potential in the dark. A two-compartment (outer and inner segment) physiological model based on known ionic mechanisms revealed that the abnormal Kv2.1-/- rod photoresponses arise principally from the voltage dependencies of the known conductances and the NCKX exchanger, and a highly elevated fraction of inward current carried by Ca2+ through CNG channels due to the aberrant depolarization. Kv2.1-/- rods had shorter outer segments than WT and dysmorphic mitochondria in their inner segments. Optical coherence tomography of knockout animals demonstrated a slow photoreceptor degeneration over a period of 6 mo. Overall, these findings reveal that Kv2.1 channels carry 70-80% of the non-NKX outward dark current of the mouse rod, and that the depolarization caused by the loss of Kv2.1 results in elevated Ca2+ influx through CNG channels and elevated free intracellular Ca2+, leading to progressive degeneration.
© 2021 Fortenbach et al.
Figures







Similar articles
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells.J Gen Physiol. 1994 Nov;104(5):909-40. doi: 10.1085/jgp.104.5.909. J Gen Physiol. 1994. PMID: 7876828 Free PMC article.
-
Membrane conductances of mouse cone photoreceptors.J Gen Physiol. 2020 Mar 2;152(3):e201912520. doi: 10.1085/jgp.201912520. J Gen Physiol. 2020. PMID: 31986199 Free PMC article.
-
GUCY2D Cone-Rod Dystrophy-6 Is a "Phototransduction Disease" Triggered by Abnormal Calcium Feedback on Retinal Membrane Guanylyl Cyclase 1.J Neurosci. 2018 Mar 21;38(12):2990-3000. doi: 10.1523/JNEUROSCI.2985-17.2018. Epub 2018 Feb 12. J Neurosci. 2018. PMID: 29440533 Free PMC article.
-
Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates.Cell Calcium. 1995 Oct;18(4):285-300. doi: 10.1016/0143-4160(95)90025-x. Cell Calcium. 1995. PMID: 8556768 Review.
Cited by
-
Clarifying the composition of the ATP consumption factors required for maintaining ion homeostasis in mouse rod photoreceptors.Sci Rep. 2023 Aug 29;13(1):14161. doi: 10.1038/s41598-023-40663-y. Sci Rep. 2023. PMID: 37644037 Free PMC article.
-
Retinoschisin and novel Na/K-ATPase interaction partners Kv2.1 and Kv8.2 define a growing protein complex at the inner segments of mammalian photoreceptors.Cell Mol Life Sci. 2022 Jul 25;79(8):448. doi: 10.1007/s00018-022-04409-9. Cell Mol Life Sci. 2022. PMID: 35876901 Free PMC article.
-
A hybrid stochastic/deterministic model of single photon response and light adaptation in mouse rods.Comput Struct Biotechnol J. 2021 Jun 23;19:3720-3734. doi: 10.1016/j.csbj.2021.06.033. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34285774 Free PMC article.
-
Analysis of the relationship between rod cell membrane currents and the photoreceptor component of electroretinograms using a cable model.Sci Rep. 2025 Apr 2;15(1):11211. doi: 10.1038/s41598-025-95998-5. Sci Rep. 2025. PMID: 40175484 Free PMC article.
-
Atp1b2Atp1b1 Knock-In Mice Exhibit a Cone-Rod Dystrophy-Like Phenotype.Cells. 2025 Jun 11;14(12):878. doi: 10.3390/cells14120878. Cells. 2025. PMID: 40558505 Free PMC article.
References
-
- Aleman, T.S., LaVail M.M., Montemayor R., Ying G., Maguire M.M., Laties A.M., Jacobson S.G., and Cideciyan A.V.. 2001. Augmented rod bipolar cell function in partial receptor loss: an ERG study in P23H rhodopsin transgenic and aging normal rats. Vision Res. 41:2779–2797. 10.1016/S0042-6989(01)00157-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

