Ambient Light Regulates Retinal Dopamine Signaling and Myopia Susceptibility
- PMID: 33502461
- PMCID: PMC7846952
- DOI: 10.1167/iovs.62.1.28
Ambient Light Regulates Retinal Dopamine Signaling and Myopia Susceptibility
Abstract
Purpose: Exposure to high-intensity or outdoor lighting has been shown to decrease the severity of myopia in both human epidemiological studies and animal models. Currently, it is not fully understood how light interacts with visual signaling to impact myopia. Previous work performed in the mouse retina has demonstrated that functional rod photoreceptors are needed to develop experimentally-induced myopia, alluding to an essential role for rod signaling in refractive development.
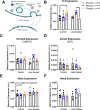
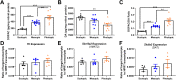
Methods: To determine whether dim rod-dominated illuminance levels influence myopia susceptibility, we housed male C57BL/6J mice under 12:12 light/dark cycles with scotopic (1.6 × 10-3 candela/m2), mesopic (1.6 × 101 cd/m2), or photopic (4.7 × 103 cd/m2) lighting from post-natal day 23 (P23) to P38. Half the mice received monocular exposure to -10 diopter (D) lens defocus from P28-38. Molecular assays to measure expression and content of DA-related genes and protein were conducted to determine how illuminance and lens defocus alter dopamine (DA) synthesis, storage, uptake, and degradation and affect myopia susceptibility in mice.
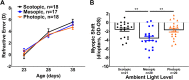
Results: We found that mice exposed to either scotopic or photopic lighting developed significantly less severe myopic refractive shifts (lens treated eye minus contralateral eye; -1.62 ± 0.37D and -1.74 ± 0.44D, respectively) than mice exposed to mesopic lighting (-3.61 ± 0.50D; P < 0.005). The 3,4-dihydroxyphenylacetic acid /DA ratio, indicating DA activity, was highest under photopic light regardless of lens defocus treatment (controls: 0.09 ± 0.011 pg/mg, lens defocus: 0.08 ± 0.008 pg/mg).
Conclusions: Lens defocus interacted with ambient conditions to differentially alter myopia susceptibility and DA-related genes and proteins. Collectively, these results show that scotopic and photopic lighting protect against lens-induced myopia, potentially indicating that a broad range of light levels are important in refractive development.
Conflict of interest statement
Disclosure:
Figures






Similar articles
-
Dim Light Exposure and Myopia in Children.Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):4804-4811. doi: 10.1167/iovs.18-24415. Invest Ophthalmol Vis Sci. 2018. PMID: 30347074 Free PMC article.
-
Ambient illuminance, retinal dopamine release and refractive development in chicks.Exp Eye Res. 2012 Oct;103:33-40. doi: 10.1016/j.exer.2012.08.004. Epub 2012 Aug 21. Exp Eye Res. 2012. PMID: 22960317
-
Short-Wavelength (Violet) Light Protects Mice From Myopia Through Cone Signaling.Invest Ophthalmol Vis Sci. 2020 Feb 7;61(2):13. doi: 10.1167/iovs.61.2.13. Invest Ophthalmol Vis Sci. 2020. PMID: 32049342 Free PMC article.
-
An updated view on the role of dopamine in myopia.Exp Eye Res. 2013 Sep;114:106-19. doi: 10.1016/j.exer.2013.02.007. Epub 2013 Feb 19. Exp Eye Res. 2013. PMID: 23434455 Review.
-
Vision and night driving abilities of elderly drivers.Traffic Inj Prev. 2013;14(5):477-85. doi: 10.1080/15389588.2012.727510. Traffic Inj Prev. 2013. PMID: 23683029 Review.
Cited by
-
Light Signaling and Myopia Development: A Review.Ophthalmol Ther. 2022 Jun;11(3):939-957. doi: 10.1007/s40123-022-00490-2. Epub 2022 Mar 11. Ophthalmol Ther. 2022. PMID: 35275382 Free PMC article. Review.
-
Myopia and daylight-A combination of factors.Front Med (Lausanne). 2025 Jul 2;12:1481209. doi: 10.3389/fmed.2025.1481209. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40672821 Free PMC article.
-
Assessment of potential myopia risk factors, including chronotype, in Estonian adolescents: a cross-sectional study.BMC Ophthalmol. 2024 Nov 5;24(1):486. doi: 10.1186/s12886-024-03747-5. BMC Ophthalmol. 2024. PMID: 39501181 Free PMC article.
-
Light Inhibits Lens-Induced Myopia through an Intensity-Dependent Dopaminergic Mechanism.Ophthalmol Sci. 2025 Mar 28;5(5):100779. doi: 10.1016/j.xops.2025.100779. eCollection 2025 Sep-Oct. Ophthalmol Sci. 2025. PMID: 40469896 Free PMC article.
-
Mice Lacking Gpr179 with Complete Congenital Stationary Night Blindness Are a Good Model for Myopia.Int J Mol Sci. 2022 Dec 22;24(1):219. doi: 10.3390/ijms24010219. Int J Mol Sci. 2022. PMID: 36613663 Free PMC article.
References
-
- Vitale S, Sperduto RD, Ferris FL 3rd. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009; 127: 1632–1639. - PubMed
-
- Wildsoet CF, Chia A, Cho P, et al. .. IMI - Interventions Myopia Institute: Interventions for Controlling Myopia Onset and Progression Report. Invest Ophthalmol Vis Sci. 2019; 60: M106–M131. - PubMed
-
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K.. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002; 43: 3633–3640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

