PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis
- PMID: 33502468
- PMCID: PMC7899060
- DOI: 10.1210/endocr/bqab015
PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis
Abstract
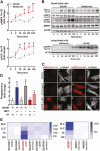
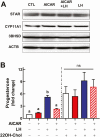
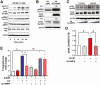
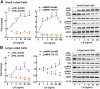
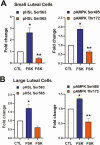
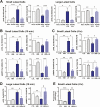
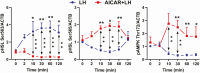
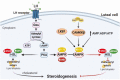
Luteinizing hormone (LH) via protein kinase A (PKA) triggers ovulation and formation of the corpus luteum, which arises from the differentiation of follicular granulosa and theca cells into large and small luteal cells, respectively. The small and large luteal cells produce progesterone, a steroid hormone required for establishment and maintenance of pregnancy. We recently reported on the importance of hormone-sensitive lipase (HSL, also known as LIPE) and lipid droplets for appropriate secretory function of the corpus luteum. These lipid-rich intracellular organelles store cholesteryl esters, which can be hydrolyzed by HSL to provide cholesterol, the main substrate necessary for progesterone synthesis. In the present study, we analyzed dynamic posttranslational modifications of HSL mediated by PKA and AMP-activated protein kinase (AMPK) as well as their effects on steroidogenesis in luteal cells. Our results revealed that AMPK acutely inhibits the stimulatory effects of LH/PKA on progesterone production without reducing levels of STAR, CYP11A1, and HSD3B proteins. Exogenous cholesterol reversed the negative effects of AMPK on LH-stimulated steroidogenesis, suggesting that AMPK regulates cholesterol availability in luteal cells. AMPK evoked inhibitory phosphorylation of HSL (Ser565). In contrast, LH/PKA decreased phosphorylation of AMPK at Thr172, a residue required for its activation. Additionally, LH/PKA increased phosphorylation of HSL at Ser563, which is crucial for enzyme activation, and decreased inhibitory phosphorylation of HSL at Ser565. The findings indicate that LH and AMPK exert opposite posttranslational modifications of HSL, presumptively regulating cholesterol availability for steroidogenesis.
Keywords: corpus luteum; hormone-sensitive lipase; lipid droplets; luteinizing hormone; progesterone; protein kinase.
Published by Oxford University Press on behalf of the Endocrine Society 2021.
Figures
Similar articles
-
Trafficking of cholesterol from lipid droplets to mitochondria in bovine luteal cells: Acute control of progesterone synthesis.FASEB J. 2020 Aug;34(8):10731-10750. doi: 10.1096/fj.202000671R. Epub 2020 Jul 2. FASEB J. 2020. PMID: 32614098 Free PMC article.
-
Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase.Endocrinology. 2010 Jun;151(6):2846-57. doi: 10.1210/en.2009-1032. Epub 2010 Mar 29. Endocrinology. 2010. PMID: 20351317 Free PMC article.
-
Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum.Int J Mol Sci. 2021 Sep 15;22(18):9972. doi: 10.3390/ijms22189972. Int J Mol Sci. 2021. PMID: 34576135 Free PMC article. Review.
-
Progesterone secretion by luteinizing human granulosa cells: a possible cAMP-dependent but PKA-independent mechanism involved in its regulation.J Endocrinol. 2004 Oct;183(1):51-60. doi: 10.1677/joe.1.05550. J Endocrinol. 2004. PMID: 15525573
-
Molecular control of luteal secretion of progesterone.Reproduction. 2002 Mar;123(3):333-9. doi: 10.1530/rep.0.1230333. Reproduction. 2002. PMID: 11882010 Review.
Cited by
-
The Role of Primary Cilia in Modulating the Luteinization Process of Ovarian Granulosa Cells in Mice.Int J Mol Sci. 2025 Feb 27;26(5):2138. doi: 10.3390/ijms26052138. Int J Mol Sci. 2025. PMID: 40076758 Free PMC article.
-
Multi-omics reveals the mechanism of Trimethylamine N-oxide derived from gut microbiota inducing liver fatty of dairy cows.BMC Genomics. 2025 Jan 6;26(1):10. doi: 10.1186/s12864-024-11067-7. BMC Genomics. 2025. PMID: 39762777 Free PMC article.
-
Semaglutide and human reproduction: caution at the intersection of energy balance, ovarian function, and follicular development.Reprod Biol Endocrinol. 2025 Aug 8;23(1):116. doi: 10.1186/s12958-025-01435-7. Reprod Biol Endocrinol. 2025. PMID: 40781307 Free PMC article. Review.
-
SFRP4 promotes autophagy and blunts FSH responsiveness through inhibition of AKT signaling in ovarian granulosa cells.Cell Commun Signal. 2024 Aug 14;22(1):396. doi: 10.1186/s12964-024-01736-1. Cell Commun Signal. 2024. PMID: 39138534 Free PMC article.
-
BAP31 depletion inhibited adipogenesis, repressed lipolysis and promoted lipid droplets abnormal growth via attenuating Perilipin1 proteasomal degradation.Int J Biol Sci. 2023 Mar 13;19(6):1713-1730. doi: 10.7150/ijbs.82178. eCollection 2023. Int J Biol Sci. 2023. PMID: 37063427 Free PMC article.
References
-
- Berisha B, Schams D. Ovarian function in ruminants. Domest Anim Endocrinol. 2005;29(2):305-317. - PubMed
-
- Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim. 2008;43(Suppl 2):260-267. - PubMed
-
- Lonergan P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology. 2011;76(9):1594-1601. - PubMed
-
- Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci. 2002;7:d1949-d1978. - PubMed
-
- McCracken JA, Carlson JC, Glew ME, et al. Prostaglandin F 2 identified as a luteolytic hormone in sheep. Nat New Biol. 1972;238(83):129-134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

