EQ-5D-3L full health state discriminates between drug and placebo in clinical trials of systemic lupus erythematosus
- PMID: 33502473
- PMCID: PMC8487305
- DOI: 10.1093/rheumatology/keab080
EQ-5D-3L full health state discriminates between drug and placebo in clinical trials of systemic lupus erythematosus
Abstract
Objectives: The objectives of this study were to investigate the discriminative ability of EQ-5D-3L full health state (FHS) in clinical trials of SLE, and to identify factors associated with FHS after treatment.
Methods: Data from the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials of belimumab (N = 1684) were utilized. FHS was defined as a response of no problems in all five EQ-5D-3L dimensions, yielding an index score of 1. The Pearson's χ2 or Fisher's exact test was employed for comparisons, and logistic regression for adjustments and assessment of independence.
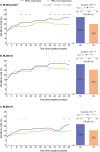
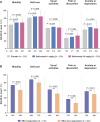
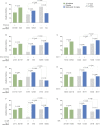
Results: We demonstrated higher EQ-5D-3L FHS frequencies among patients given standard therapy (ST) plus the licensed belimumab dose vs ST alone (26.1% vs 19.4%; P = 0.001; week 52), and within SRI-4 responders vs non-responders (27.0% vs 19.8%; P < 0.001; week 52) from weeks 36 to 52. In multivariable regression analysis, SLEDAI-2K (OR: 0.90; 95% CI: 0.87, 0.94; P < 0.001) and SLICC/ACR Damage Index (OR: 0.79; 95% CI: 0.69, 0.91; P = 0.001) scores were independently associated with lower FHS frequencies at week 52, while adding monthly infusions of belimumab 10 mg/kg to ST favoured FHS perception (OR: 1.60; 95% CI: 1.15, 2.24; P = 0.006). Add-on belimumab 10 mg/kg yielded higher FHS frequencies in antimalarial users vs non-users (29.9% vs 20.1%; P = 0.011), and in anti-dsDNA- and anti-Sm- positive vs negative patients (31.4% vs 13.4%; P < 0.001 and 33.0% vs 22.6%; P = 0.010, respectively), whereas no significant differences were observed in patients given ST alone.
Conclusion: EQ-5D-3L FHS distinguished belimumab from placebo and responders from non-responders, and exhibited known-group validity in subgroup analysis. FHS may prove a useful patient-reported outcome in SLE studies.
Keywords: health-related quality of life; outcomes research; patient perspective; patient-reported outcomes; systemic lupus erythematosus.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Jolly M.How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–8. - PubMed
-
- Annapureddy N, Devilliers H, Jolly M.. Patient-reported outcomes in lupus clinical trials with biologics. Lupus 2016;25:1111–21. - PubMed
-
- Strand V, Gladman D, Isenberg D. et al. Endpoints: consensus recommendations from OMERACT IV. Outcome Measures in Rheumatology. Lupus 2000;9:322–7. - PubMed
-
- Yen JC, Neville C, Fortin PR.. Discordance between patients and their physicians in the assessment of lupus disease activity: relevance for clinical trials. Lupus 1999;8:660–70. - PubMed
-
- Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GlaxoSmithKline Investigator-Sponsored Studies (ISS) programme, and grants from the Swedish Rheumatism Association (R-932236)
- FAI-2019-0635/King Gustaf V's 80-year Foundation
- 2019-00290/Professor Nanna Svartz Foundation
- 2019-12/Ulla and Roland Gustafsson Foundation
- Region Stockholm and Karolinska Institutet
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

