Evaluation of Ruxolitinib for Steroid-Refractory Chronic Graft-vs-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation
- PMID: 33502484
- PMCID: PMC7841467
- DOI: 10.1001/jamanetworkopen.2020.34750
Evaluation of Ruxolitinib for Steroid-Refractory Chronic Graft-vs-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
Importance: Ruxolitinib, a selective inhibitor of the Janus kinases 1/2 signaling pathway, has shown a significant response in steroid-refractory chronic graft-vs-host disease (SR-cGVHD), a major cause of morbidity and mortality in individuals who have undergone allogeneic hematopoietic stem cell transplantation (HSCT).
Objectives: To investigate the clinical response to ruxolitinib in patients with SR-cGVHD after allogeneic HSCT and to evaluate its safety profile during the treatment course.
Design, setting, and participants: This single-center case series included 41 consecutive patients who were treated with ruxolitinib for SR-cGVHD after allogeneic HSCT between August 2017 and December 2019. Data were collected from each patient's medical record at the First Affiliated Hospital of Zhejiang University School of Medicine. Data analysis was conducted from March to May 2020.
Exposure: Ruxolitinib.
Main outcomes and measures: Treatment responses, factors associated with response, and adverse effects during ruxolitinib administration.
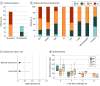
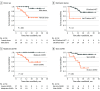
Findings: Overall, 41 patients (median [range] age, 31 [17-56] years; 14 [34.1%] women) were treated with ruxolitinib and included in this study. A total of 15 patients (36.6%) had a complete remission, and 14 (34.1%) had a partial remission, with an overall response rate of 70.7% (29 patients; 95% CI, 56.2%-85.3%). Lung involvement (odds ratio, 0.112; 95% CI, 0.020-0.639; P = .01) and matched related donors (odds ratio, 0.149; 95% CI, 0.022-0.981; P = .048) were associated with less favorable treatment response. Major adverse events associated with ruxolitinib were cytopenias and infectious complications. The median (range) follow-up for this cohort was 14.9 (1.4-32.5) months. Prolonged survival was observed in patients with a male donor (P = .006), complete remission before transplantation (P = .02), baseline moderate cGVHD (P = .02), and skin cGVHD (P = .001).
Conclusions and relevance: In this small, single-site case series, ruxolitinib demonstrated a significant response in heavily pretreated patients with SR-cGVHD and a reasonably well-tolerated safety profile. The results add to the body of literature suggesting ruxolitinib as a promising treatment option in SR-cGVHD.
Conflict of interest statement
Figures

Comment in
-
Ruxolitinib for the Treatment of Steroid-Refractory Chronic Graft-vs-Host Disease-Another Hopeful Step Forward.JAMA Netw Open. 2021 Jan 4;4(1):e2035719. doi: 10.1001/jamanetworkopen.2020.35719. JAMA Netw Open. 2021. PMID: 33502479 No abstract available.
Similar articles
-
Efficiency and Toxicity of Ruxolitinib as a Salvage Treatment for Steroid-Refractory Chronic Graft-Versus-Host Disease.Front Immunol. 2021 Jun 30;12:673636. doi: 10.3389/fimmu.2021.673636. eCollection 2021. Front Immunol. 2021. PMID: 34276662 Free PMC article.
-
Treatment of steroid-refractory acute/chronic graft versus host disease: A single-center real-world experience of ruxolitinib in combination with extracorporeal photopheresis in a high-risk population.Leuk Res. 2024 Dec;147:107611. doi: 10.1016/j.leukres.2024.107611. Epub 2024 Oct 29. Leuk Res. 2024. PMID: 39500129
-
Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey.Leukemia. 2015 Oct;29(10):2062-8. doi: 10.1038/leu.2015.212. Epub 2015 Jul 31. Leukemia. 2015. PMID: 26228813 Free PMC article.
-
Efficacy and safety of ruxolitinib for steroid-refractory graft-versus-host disease: Systematic review and meta-analysis of randomised and non-randomised studies.PLoS One. 2022 Jul 29;17(7):e0271979. doi: 10.1371/journal.pone.0271979. eCollection 2022. PLoS One. 2022. PMID: 35905125 Free PMC article.
-
Ruxolitinib Treatment of Steroid-Refractory Graft-versus-Host Disease in Children: A Case Series and Review of the Literature.Paediatr Drugs. 2023 Sep;25(5):577-584. doi: 10.1007/s40272-023-00577-8. Epub 2023 Jun 7. Paediatr Drugs. 2023. PMID: 37284944 Review.
Cited by
-
Ruxolitinib for Treatment of Steroid-Refractory Graft-versus-Host Disease: Real-World Data from Chinese Patients.Drug Des Devel Ther. 2021 Nov 30;15:4875-4883. doi: 10.2147/DDDT.S338752. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34880598 Free PMC article.
-
Long-term follow-up results of ruxolitinib as salvage therapy for chronic graft-versus-host disease.Hematol Transfus Cell Ther. 2025 Jul-Sep;47(3):103835. doi: 10.1016/j.htct.2025.103835. Epub 2025 May 11. Hematol Transfus Cell Ther. 2025. PMID: 40354779 Free PMC article.
-
Ruxolitinib does not completely abrogate the functional capabilities of TLR4/9 ligand-activated NK cells.Front Immunol. 2023 Jan 5;13:1045316. doi: 10.3389/fimmu.2022.1045316. eCollection 2022. Front Immunol. 2023. PMID: 36685552 Free PMC article.
-
Treatment patterns of extracorporeal photopheresis in steroid-refractory graft versus host disease: A delphi study.Bone Marrow Transplant. 2025 Jul 22. doi: 10.1038/s41409-025-02687-y. Online ahead of print. Bone Marrow Transplant. 2025. PMID: 40696076 No abstract available.
-
Propensity score matching analysis comparing the efficacy of Ruxolitinib to historical controls in second-line or beyond treatment for chronic GvHD after steroid failure.Bone Marrow Transplant. 2023 Sep;58(9):1024-1032. doi: 10.1038/s41409-023-02020-5. Epub 2023 Jun 26. Bone Marrow Transplant. 2023. PMID: 37365296
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

