Identification of CXCL11 as part of chemokine network controlling skeletal muscle development
- PMID: 33502606
- PMCID: PMC8141492
- DOI: 10.1007/s00441-020-03398-0
Identification of CXCL11 as part of chemokine network controlling skeletal muscle development
Abstract
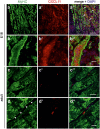
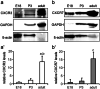
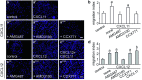
The chemokine, CXCL12, and its receptors, CXCR4 and CXCR7, play pivotal roles during development and maintenance of limb muscles. CXCR7 additionally binds CXCL11, which uses CXCR3 as its prime receptor. Based on this cross-talk, we investigate whether CXCL11 would likewise affect development and/or function of skeletal muscles. Western blotting and immunolabelling demonstrated the developmentally restricted expression of CXCL11 in rat limb muscles, which was contrasted by the continuous expression of its receptors in proliferating and differentiating C2C12 cells as well as in late embryonic to adult rat limb muscle fibres. Consistent with a prime role in muscle formation, functional studies identified CXCL11 as a potent chemoattractant for undifferentiated C2C12 cells and further showed that CXCL11 does neither affect myoblast proliferation and differentiation nor metabolic/catabolic pathways in formed myotubes. The use of selective receptor antagonists unravelled complementary effects of CXCL11 and CXCL12 on C2C12 cell migration, which either require CXCR3/CXCR7 or CXCR4, respectively. Our findings provide new insights into the chemokine network controlling skeletal muscle development and function and, thus, might provide a base for future therapies of muscular diseases.
Keywords: CXCL11; CXCR3; CXCR7; Myoblasts; Skeletal muscle fibres.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Common structural interactions between the receptors CXCR3, CXCR4 and CXCR7 complexed with their natural ligands, CXCL11 and CXCL12, by a modeling approach.Cytokine. 2013 Oct;64(1):316-21. doi: 10.1016/j.cyto.2013.05.024. Epub 2013 Jun 15. Cytokine. 2013. PMID: 23773308
-
Expression and function of the SDF-1 chemokine receptors CXCR4 and CXCR7 during mouse limb muscle development and regeneration.Exp Cell Res. 2012 Oct 15;318(17):2178-90. doi: 10.1016/j.yexcr.2012.06.020. Epub 2012 Jul 3. Exp Cell Res. 2012. PMID: 22766125
-
Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12.Cytokine Growth Factor Rev. 2013 Feb;24(1):41-9. doi: 10.1016/j.cytogfr.2012.08.007. Epub 2012 Sep 16. Cytokine Growth Factor Rev. 2013. PMID: 22989616 Free PMC article. Review.
-
Interactions of the chemokines CXCL11 and CXCL12 in human tumor cells.BMC Cancer. 2022 Dec 20;22(1):1335. doi: 10.1186/s12885-022-10451-4. BMC Cancer. 2022. PMID: 36539774 Free PMC article.
-
CXCR7 impact on CXCL12 biology and disease.Trends Mol Med. 2013 Jan;19(1):12-22. doi: 10.1016/j.molmed.2012.10.004. Epub 2012 Nov 12. Trends Mol Med. 2013. PMID: 23153575 Review.
Cited by
-
Evaluation of I-TAC as a potential early plasma marker to differentiate between critical and non-critical COVID-19.Cell Stress. 2021 Dec 21;6(1):6-16. doi: 10.15698/cst2022.01.262. eCollection 2022 Jan. Cell Stress. 2021. PMID: 35083423 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

