Low Incidence of Opioid-Induced Respiratory Depression Observed with Oliceridine Regardless of Age or Body Mass Index: Exploratory Analysis from a Phase 3 Open-Label Trial in Postsurgical Pain
- PMID: 33502739
- PMCID: PMC8119589
- DOI: 10.1007/s40122-020-00232-x
Low Incidence of Opioid-Induced Respiratory Depression Observed with Oliceridine Regardless of Age or Body Mass Index: Exploratory Analysis from a Phase 3 Open-Label Trial in Postsurgical Pain
Abstract
Introduction: Advanced age and obesity are reported to increase the risk of opioid-induced respiratory depression (OIRD). Oliceridine, an intravenous opioid, is a G-protein-biased agonist at the µ-opioid receptor that may provide improved safety. The recent phase 3 ATHENA open-label, multicenter study evaluated postoperative use of oliceridine in patients with moderate-to-severe acute pain. This exploratory analysis of the ATHENA data examined the incidence of OIRD in older (≥ 65 years) and/or obese (BMI ≥ 30 kg/m2) patients and analyzed risk factors of OIRD.
Methods: Patients aged ≥ 18 years with a score ≥ 4 on an 11-point numeric pain rating scale (NPRS) received IV oliceridine as needed via bolus dosing and/or patient-controlled analgesia (PCA). OIRD occurring within 48 h of last dose of oliceridine was defined using two established definitions: (1) naloxone use, (2) respiratory rate < 10 breaths per minute and/or oxygen saturation < 90%.
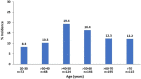
Results: A total of 724 surgical patients with a mean age of 54.5 ± 15.9 years and a mean NRS score of 6.2 ± 2.1 were included in this analysis; 33.3% (241/724) were ≥ 65 years of age and 46.3% (335/724) had BMI (body mass index) ≥ 30 kg/m2. The overall OIRD incidence was 13.7% with no patients requiring naloxone. The OIRD incidence was similar in the elderly and younger adults' cohorts [10.8 vs. 15.1%, OR 0.68 (0.42, 1.1), p = 0.11], and in obese and non-obese groups [14.0 vs. 13.4%, OR 1.06 (0.69, 1.62), p = 0.80]. In patients that were both elderly and obese (n = 120), the incidence was 10.8%. The multivariate analysis identified baseline NRS ≥ 6 [OR 1.6 (1.0, 2.4), p = 0.0499], PCA administration [OR 1.9 (1.2, 3.1), p = 0.005], and concomitant use of benzodiazepines and/or gabapentinoids [OR 1.6 (1.0, 2.6), p = 0.045], as being associated with OIRD.
Conclusions: Postoperative oliceridine use in patients with advanced age and/or increased BMI was not associated with increased risk of OIRD.
Keywords: Analgesia; Biased opioid; Oliceridine; Postoperative pain; Respiratory depression.
Figures


References
-
- Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain Off J Am Pain Soc. 2016;17(2):131–157. - PubMed
-
- Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010;11(12):1859–1871. - PubMed
-
- Stein C. New concepts in opioid analgesia. Expert Opin Investig Drugs. 2018;27(10):765–775. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

