Decreased circUBAP2 Expression Is Associated with Preeclampsia by Limiting Trophoblast Cell Proliferation and Migration
- PMID: 33502747
- PMCID: PMC8289767
- DOI: 10.1007/s43032-020-00450-w
Decreased circUBAP2 Expression Is Associated with Preeclampsia by Limiting Trophoblast Cell Proliferation and Migration
Abstract
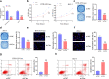
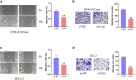
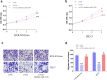
Preeclampsia (PE) is a common obstetric disease and a major cause of maternal, newborn, and fetal death. This condition is a multisystem disorder characterized by hypertension, proteinuria, and involvement of the kidney, liver, and nervous system. It is generally believed that the placenta is the main cause of PE. circRNAs are a special class of noncoding RNAs that can form covalently closed continuous ring structures with tissue-specific conservation, and they have been reported to play a wide range of regulatory functions in various diseases, including PE. In this study, we reported a novel circUBAP2 (hsa_circ_0003496) and found that it was downregulated in placental tissues from patients with PE compared to healthy controls. After knocking down circUBAP2 in trophoblast cells, we found that cell proliferation and migration were significantly suppressed. In addition, preliminary mechanistic studies showed that circUBAP2 can sponge miR-1244, and FOXM1 was identified as a target gene for miR-1244. Cotransfection of si-circUBAP2 and a miR-1244 inhibitor partially reversed the suppressive effect induced by circUBAP2 depletion on proliferation and migration. In conclusion, the circUBAP2/miR-1244/FOXM1 axis might be a promising molecular marker for the diagnosis and treatment of PE.
Keywords: FOXM1; Placenta; Preeclampsia; circUBAP2; miR-1244.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Circ_0037078 promotes trophoblast cell proliferation, migration, invasion and angiogenesis by miR-576-5p/IL1RAP axis.Am J Reprod Immunol. 2022 Jan;87(1):e13507. doi: 10.1111/aji.13507. Epub 2021 Dec 2. Am J Reprod Immunol. 2022. PMID: 34724268
-
Circ_0015382 is associated with preeclampsia and regulates biological behaviors of trophoblast cells through miR-149-5p/TFPI2 axis.Placenta. 2021 May;108:73-80. doi: 10.1016/j.placenta.2021.03.005. Epub 2021 Mar 27. Placenta. 2021. PMID: 33819864
-
Circ_0001438 participates in the pathogenesis of preeclampsia via the circ_0001438/miR-942/NLRP3 regulatory network.Placenta. 2021 Jan 15;104:40-50. doi: 10.1016/j.placenta.2020.11.005. Epub 2020 Nov 18. Placenta. 2021. PMID: 33253995
-
Research Progress on Extracellular Matrix Involved in the Development of Preeclampsia.Curr Protein Pept Sci. 2024;25(7):527-538. doi: 10.2174/0113892037284176240302052521. Curr Protein Pept Sci. 2024. PMID: 38561606 Review.
-
Role of Circular RNAs in Preeclampsia.Dis Markers. 2019 May 2;2019:7237495. doi: 10.1155/2019/7237495. eCollection 2019. Dis Markers. 2019. PMID: 31191755 Free PMC article. Review.
Cited by
-
Current understanding of circular RNAs in preeclampsia.Hypertens Res. 2024 Jun;47(6):1607-1619. doi: 10.1038/s41440-024-01675-x. Epub 2024 Apr 11. Hypertens Res. 2024. PMID: 38605141 Review.
-
Exploration of adverse events associated with risdiplam use: Retrospective cases from the US Food and Drug Administration Adverse Event Reporting System (FAERS) database.PLoS One. 2024 Mar 1;19(3):e0298609. doi: 10.1371/journal.pone.0298609. eCollection 2024. PLoS One. 2024. PMID: 38427665 Free PMC article.
-
Circular RNAs in Pregnancy and the Placenta.Int J Mol Sci. 2022 Apr 20;23(9):4551. doi: 10.3390/ijms23094551. Int J Mol Sci. 2022. PMID: 35562943 Free PMC article. Review.
-
The circRNA Landscape in Recurrent Pregnacy Loss (RPL): A Comparison of Four Reproductive Tissues.Int J Mol Sci. 2024 Nov 25;25(23):12622. doi: 10.3390/ijms252312622. Int J Mol Sci. 2024. PMID: 39684334 Free PMC article.
-
The circular RNA circ_0099630/miR-940/receptor-associated factor 6 regulation cascade modulates the pathogenesis of periodontitis.J Dent Sci. 2022 Oct;17(4):1566-1576. doi: 10.1016/j.jds.2022.04.005. Epub 2022 Apr 25. J Dent Sci. 2022. PMID: 36299308 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

