Time response of rat testicular alterations induced by cryptorchidism and orchiopexy
- PMID: 33502821
- PMCID: PMC7839953
- DOI: 10.1111/iep.12384
Time response of rat testicular alterations induced by cryptorchidism and orchiopexy
Abstract
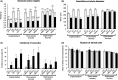
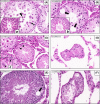
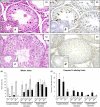
Cryptorchidism is one of the main risk factors for infertility and testicular cancer. Orchiopexy surgery corrects cryptorchidism effects. Different models of cryptorchidism developed in the rat include surgery. We assessed testicular alterations in rats submitted to surgical cryptorchidism and examined their potential for reversibility at different time points in order to verify time dependency effect(s) on the recovery of the undescended testes. Cryptorchidism was induced in 3-week-old rats. Animals were euthanized 3, 6 or 11 weeks after surgery to evaluate the morphological progression of cryptorchidism-induced germinative epithelial alterations. Other groups underwent orchiopexy 3, 5 or 9 weeks after surgical cryptorchidism, before or after puberty. Animals were euthanized 3 or 8 weeks after orchiopexy. Controls underwent sham surgery at the same time points as the surgical groups. Cryptorchid testes showed decreased weight, germinative epithelial degeneration, apoptosis and vacuolation, corresponding to impairment of spermatogenesis and of Sertoli cells. Some tubules has a Sertoli cell-only pattern and atrophy. The intensity of damage was related to the duration of cryptorchidism. After orchiopexy, spermatogenesis completely recovered only when testicular relocation occurred before puberty and the interval for recovery was extended. These results indicate that age, sexual maturity and extension of germ cell damage were relevant for producing germ cell restoration and normal spermatogenesis. We provide original observations on the time dependency of testicular alterations induced by cryptorchidism and their restoration using morphologic, morphometric and immunohistochemical approaches. It may be useful to study germ cell impairment, progression and recovery in different experimental settings, including exposure to exogenous chemicals.
Keywords: cryptorchidism; germ cell damage; orchiopexy; testicular damage.
© 2020 Company of the International Journal of Experimental Pathology (CIJEP).
Conflict of interest statement
There are no potential conflicts of interest. During the development of this study, authors APFC, LMMG, NPS and MGNP were enrolled in the Graduate Program of Pathology at the UNESP Medical School, Botucatu, SP, Brazil.
Figures


Similar articles
-
Experimental cryptorchidism enhances testicular susceptibility to dibutyl phthalate or acrylamide in Sprague-Dawley rats.Hum Exp Toxicol. 2019 Aug;38(8):899-913. doi: 10.1177/0960327119845040. Epub 2019 Apr 17. Hum Exp Toxicol. 2019. PMID: 30995857
-
Early orchiopexy improves subsequent testicular development and spermatogenesis in the experimental cryptorchid rat model.J Urol. 2008 Mar;179(3):1195-9. doi: 10.1016/j.juro.2007.10.029. J Urol. 2008. PMID: 18206164
-
A quantitative (stereological) study of the effects of experimental unilateral cryptorchidism and subsequent orchiopexy on spermatogenesis in adult rabbit testis.Reproduction. 2002 Jul;124(1):95-105. doi: 10.1530/rep.0.1240095. Reproduction. 2002. PMID: 12090923
-
Cryptorchidism --disease or symptom?Ann Endocrinol (Paris). 2014 May;75(2):72-6. doi: 10.1016/j.ando.2014.04.010. Epub 2014 Apr 29. Ann Endocrinol (Paris). 2014. PMID: 24786701 Review.
-
A narrative review of the history and evidence-base for the timing of orchidopexy for cryptorchidism.J Pediatr Urol. 2021 Apr;17(2):239-245. doi: 10.1016/j.jpurol.2021.01.013. Epub 2021 Jan 23. J Pediatr Urol. 2021. PMID: 33551366 Review.
Cited by
-
Is Human Chorionic Gonadotropin a Reliable Marker for Testicular Germ Cell Tumor? New Perspectives for a More Accurate Diagnosis.Cancers (Basel). 2025 Jul 21;17(14):2409. doi: 10.3390/cancers17142409. Cancers (Basel). 2025. PMID: 40723290 Free PMC article. Review.
-
Testicular alterations in cryptorchid/orchiopexic rats chronically exposed to acrylamide or di-butyl-phthalate.J Toxicol Pathol. 2022 Apr;35(2):159-170. doi: 10.1293/tox.2021-0045. Epub 2021 Mar 7. J Toxicol Pathol. 2022. PMID: 35516837 Free PMC article.
-
Quantification of testicular fat content: the value of evaluating testicular function after cryptorchidism surgery.Pediatr Res. 2024 Dec;96(7):1788-1793. doi: 10.1038/s41390-024-03272-7. Epub 2024 May 20. Pediatr Res. 2024. PMID: 38769403
References
-
- Skakkebaek NE, Rajpert‐De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972‐978. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

