Clinical Outcomes and Patient-Matched Molecular Composition of Relapsed Medulloblastoma
- PMID: 33502920
- PMCID: PMC8078396
- DOI: 10.1200/JCO.20.01359
Clinical Outcomes and Patient-Matched Molecular Composition of Relapsed Medulloblastoma
Abstract
Purpose: We sought to investigate clinical outcomes of relapsed medulloblastoma and to compare molecular features between patient-matched diagnostic and relapsed tumors.
Methods: Children and infants enrolled on either SJMB03 (NCT00085202) or SJYC07 (NCT00602667) trials who experienced medulloblastoma relapse were analyzed for clinical outcomes, including anatomic and temporal patterns of relapse and postrelapse survival. A largely independent, paired molecular cohort was analyzed by DNA methylation array and next-generation sequencing.
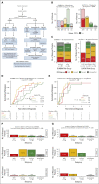
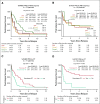
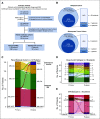
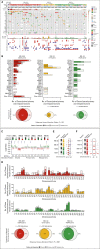
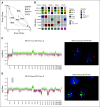
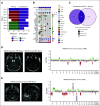
Results: A total of 72 of 329 (22%) SJMB03 and 52 of 79 (66%) SJYC07 patients experienced relapse with significant representation of Group 3 and wingless tumors. Although most patients exhibited some distal disease (79%), 38% of patients with sonic hedgehog tumors experienced isolated local relapse. Time to relapse and postrelapse survival varied by molecular subgroup with longer latencies for patients with Group 4 tumors. Postrelapse radiation therapy among previously nonirradiated SJYC07 patients was associated with long-term survival. Reirradiation was only temporizing for SJMB03 patients. Among 127 patients with patient-matched tumor pairs, 9 (7%) experienced subsequent nonmedulloblastoma CNS malignancies. Subgroup (96%) and subtype (80%) stabilities were largely maintained among the remainder. Rare subgroup divergence was observed from Group 4 to Group 3 tumors, which is coincident with genetic alterations involving MYC, MYCN, and FBXW7. Subgroup-specific patterns of alteration were identified for driver genes and chromosome arms.
Conclusion: Clinical behavior of relapsed medulloblastoma must be contextualized in terms of up-front therapies and molecular classifications. Group 4 tumors exhibit slower biological progression. Utility of radiation at relapse is dependent on patient age and prior treatments. Degree and patterns of molecular conservation at relapse vary by subgroup. Relapse tissue enables verification of molecular targets and identification of occult secondary malignancies.
Figures
References
-
- Curtin SC, Minino AM, Anderson RN: Declines in cancer death rates among children and adolescents in the United States, 1999-2014. NCHS Data Brief:1-8, 2016 - PubMed
-
- Gajjar A Chintagumpala M Ashley D, et al. : Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol 7:813-820, 2006 - PubMed
-
- Johnston DL Keene D Strother D, et al. : Survival following tumor recurrence in children with medulloblastoma. J Pediatr Hematol Oncol 40:e159-e163, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

