First insights into the structural features of Ebola virus methyltransferase activities
- PMID: 33503246
- PMCID: PMC7897494
- DOI: 10.1093/nar/gkaa1276
First insights into the structural features of Ebola virus methyltransferase activities
Abstract
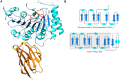
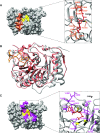
The Ebola virus is a deadly human pathogen responsible for several outbreaks in Africa. Its genome encodes the 'large' L protein, an essential enzyme that has polymerase, capping and methyltransferase activities. The methyltransferase activity leads to RNA co-transcriptional modifications at the N7 position of the cap structure and at the 2'-O position of the first transcribed nucleotide. Unlike other Mononegavirales viruses, the Ebola virus methyltransferase also catalyses 2'-O-methylation of adenosines located within the RNA sequences. Herein, we report the crystal structure at 1.8 Å resolution of the Ebola virus methyltransferase domain bound to a fragment of a camelid single-chain antibody. We identified structural determinants and key amino acids specifically involved in the internal adenosine-2'-O-methylation from cap-related methylations. These results provide the first high resolution structure of an ebolavirus L protein domain, and the framework to investigate the effects of epitranscriptomic modifications and to design possible antiviral drugs against the Filoviridae family.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Lawrence P., Danet N., Reynard O., Volchkova V., Volchkov V.. Human transmission of Ebola virus. Curr. Opin. Virol. 2017; 22:51–58. - PubMed
-
- Ollmann Saphire E. A Vaccine against Ebola Virus. Cell. 2020; 181:6. - PubMed
-
- Elliott L.H., Sanchez A., Holloway B.P., Kiley M.P., McCormick J.B.. Ebola protein analyses for the determination of genetic organization. Arch. Virol. 1993; 133:423–436. - PubMed
-
- Sanchez A., Kiley M.P., Holloway B.P., Auperin D.D.. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993; 29:215–240. - PubMed
-
- Ogino T., Banerjee A.K.. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol. Cell. 2007; 25:85–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

