First-in-human study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of ALPN-101, a dual CD28/ICOS antagonist, in healthy adult subjects
- PMID: 33503289
- PMCID: PMC8301585
- DOI: 10.1111/cts.12983
First-in-human study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of ALPN-101, a dual CD28/ICOS antagonist, in healthy adult subjects
Abstract
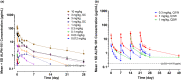
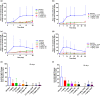
ALPN-101 (ICOSL vIgD-Fc) is an Fc fusion protein of a human inducible T cell costimulatory ligand (ICOSL) variant immunoglobulin domain (vIgD) designed to inhibit the cluster of differentiation 28 (CD28) and inducible T cell costimulator (ICOS) pathways simultaneously. A first-in-human study evaluated the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of ALPN-101 in healthy adult subjects. ALPN-101 was generally well-tolerated with no evidence of cytokine release, clinically significant immunogenicity, or severe adverse events following single subcutaneous (SC) doses up to 3 mg/kg or single intravenous (IV) doses up to 10 mg/kg or up to 4 weekly IV doses of up to 1 mg/kg. ALPN-101 exhibited a dose-dependent increase in exposure with an estimated terminal half-life of 4.3-8.6 days and SC bioavailability of 60.6% at 3 mg/kg. Minimal to modest accumulation in exposure was observed with repeated IV dosing. ALPN-101 resulted in a dose-dependent increase in maximum target saturation and duration of high-level target saturation. Consistent with its mechanism of action, ALPN-101 inhibited cytokine production in whole blood stimulated by Staphylococcus aureus enterotoxin B ex vivo, as well as antibody responses to keyhole limpet hemocyanin immunization, reflecting immunomodulatory effects upon T cell and T-dependent B cell responses, respectively. In conclusion, ALPN-101 was well-tolerated in healthy subjects with dose-dependent PK and PD consistent with the known biology of the CD28 and ICOS costimulatory pathways. Further clinical development of ALPN-101 in inflammatory and/or autoimmune diseases is therefore warranted.
© 2021 Alpine Immune Sciences, Inc. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Jing Yang, Jan L. Hillson, Gary D. Means, Russell J. Sanderson, Kay Carley, Almudena Tercero, Kristi L. Manjarrez, Jennifer R. Wiley, and Stanford L. Peng are employees of Alpine Immune Sciences. Jason D. Lickliter is a full‐time employee of Nucleus Network.
Figures


References
-
- Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co‐receptor signalling. Nat Rev Immunol. 2003;3:544‐556. - PubMed
-
- Wekerle T, Grinyo JM. Belatacept: from rational design to clinical application. Transpl Int. 2012;25:139‐150. - PubMed
-
- Orencia (abatacept) [package insert]. US Food and Drug Administration website https://wwwaccessdatafdagov/drugsatfda_docs/label/2020/125118s194s199s22... Accessed June 17, 2020.
-
- Merrill JT, Burgos‐Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non‐life‐threatening manifestations of systemic lupus erythematosus: results of a twelve‐month, multicenter, exploratory, phase IIb, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum. 2010;62:3077‐3087. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

