Pharmacokinetic equivalence of CT-P17 to high-concentration (100 mg/ml) reference adalimumab: A randomized phase I study in healthy subjects
- PMID: 33503313
- PMCID: PMC8301575
- DOI: 10.1111/cts.12967
Pharmacokinetic equivalence of CT-P17 to high-concentration (100 mg/ml) reference adalimumab: A randomized phase I study in healthy subjects
Abstract
This study aimed to demonstrate pharmacokinetic (PK) equivalence of a single dose of the proposed adalimumab biosimilar CT-P17 to United States-licensed adalimumab (US-adalimumab) and European Union-approved adalimumab (EU-adalimumab). This double-blind, parallel-group, phase I trial (clinicaltrials.gov NCT03970824) was conducted at 10 hospitals (Republic of Korea), in which healthy subjects (1:1:1) were randomized to receive a single 40 mg (100 mg/ml) subcutaneous injection of CT-P17, US-adalimumab, or EU-adalimumab. Primary end points were PK equivalence in terms of: area under the concentration-time curve from time zero to infinity (AUC0-inf ); AUC from time zero to the last quantifiable concentration (AUC0-last ); and maximum serum concentration (Cmax ). PK equivalence was concluded if 90% confidence intervals (CIs) for percent ratios of geometric least squares means (GLSMs) for pairwise comparisons were within the equivalence margin of 80-125%. Additional PK end points, safety, and immunogenicity were evaluated. Of the 312 subjects who were randomized (103 CT-P17; 103 US-adalimumab; 106 EU-adalimumab), 308 subjects received study drug. AUC0-inf , AUC0-last , and Cmax were equivalent among CT-P17, US-adalimumab, and EU-adalimumab, because 90% CIs for the ratios of GLSMs were within the 80-125% equivalence margin for each pairwise comparison. Secondary PK end points, safety, and immunogenicity were similar between treatment groups. In conclusion, PK equivalence for single-dose administration of CT-P17, EU-adalimumab, and US-adalimumab was demonstrated in healthy adults. Safety and immunogenicity profiles were comparable between treatment groups and consistent with previous reports for adalimumab biosimilars.
© 2021 Celltrion, Inc. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
K.‐S.Y., I.‐J.J., H.‐S.L., J.H.H., M.‐G.K., M.K.P., D.‐Y.C., M.S.P., J.Y.C., J.‐L.G., S.H.L., S.K.Y., and I.S.K. received investigator fees from Celltrion, Inc. in relation to this study. S.J.L., S.H.K., Y.J.B., and J.B.C. are employees of Celltrion, Inc. D.E.F. has received grant/research support from Corbus Pharmaceuticals, CSL Behring, Galapagos NV, Gilead Sciences, Inc., GlaxoSmithKline PLC, Kadmon Pharmaceuticals, LLC, Pfizer Inc., and Talaris Therapeutics; and honoraria for speaking and/or consulting from AbbVie Inc., Amgen Inc., Boehringer Ingelheim GmbH, Celltrion Healthcare Co. Ltd., Corbus Pharmaceuticals, CSL Behring, Galapagos NV, Genentech Inc., Gilead Sciences, Inc., Novartis AG, Pfizer Inc., Roche Pharmaceuticals, and Talaris Therapeutics. E.K. has received research support from Amgen Inc., Merck & Co, Inc., Pfizer Inc., and PuraPharm Corporation Ltd.; has consulting agreements and/or advisory board membership with AbbVie Inc., Amgen Inc., Bristol‐Myers Squibb Company, Celltrion Healthcare Co. Ltd., Eli Lilly and Company, F. Hoffman‐La Roche AG, Gilead Sciences, Inc., Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Myriad Autoimmune, Pfizer Inc., Samsung Bioepis Co., Ltd, Sandoz Inc., and Sanofi Genzyme; and has speaker honoraria agreements with AbbVie Inc., Amgen Inc., F. Hoffman‐La Roche AG, Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Novartis AG, Pfizer Inc., and Sanofi Genzyme. J.K. has received research support (paid to the University of Massachusetts Medical School) from Gilead Sciences, Inc. and Pfizer Inc. and honoraria for consulting from Alvotech Suisse AG, Arena Pharmaceuticals, Inc., Boehringer Ingelheim GmbH, Celltrion Healthcare Co. Ltd., Mylan Inc., Novartis AG, Samsung Bioepis Co. Ltd., and Sandoz Inc.
Figures
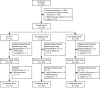
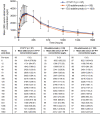
References
-
- US Food and Drug Administration . Humira prescribing information, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125057Orig1s41.... Accessed March 13, 2020.
-
- European Medicines Agency . Humira summary of product characteristics, 2020. https://www.ema.europa.eu/en/documents/product‐information/humira‐epar‐p.... Accessed March 6, 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

