Levonorgestrel vs. Copper Intrauterine Devices for Emergency Contraception
- PMID: 33503342
- PMCID: PMC7983017
- DOI: 10.1056/NEJMoa2022141
Levonorgestrel vs. Copper Intrauterine Devices for Emergency Contraception
Abstract
Background: In the United States, more intrauterine device (IUD) users select levonorgestrel IUDs than copper IUDs for long-term contraception. Currently, clinicians offer only copper IUDs for emergency contraception because data are lacking on the efficacy of the levonorgestrel IUD for this purpose.
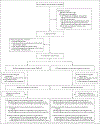
Methods: This randomized noninferiority trial, in which participants were unaware of the group assignments, was conducted at six clinics in Utah and included women who sought emergency contraception after at least one episode of unprotected intercourse within 5 days before presentation and agreed to placement of an IUD. We randomly assigned participants in a 1:1 ratio to receive a levonorgestrel 52-mg IUD or a copper T380A IUD. The primary outcome was a positive urine pregnancy test 1 month after IUD insertion. When a 1-month urine pregnancy test was unavailable, we used survey and health record data to determine pregnancy status. The prespecified noninferiority margin was 2.5 percentage points.
Results: Among the 355 participants randomly assigned to receive levonorgestrel IUDs and 356 assigned to receive copper IUDs, 317 and 321, respectively, received the interventions and provided 1-month outcome data. Of these, 290 in the levonorgestrel group and 300 in the copper IUD group had a 1-month urine pregnancy test. In the modified intention-to-treat and per-protocol analyses, pregnancy rates were 1 in 317 (0.3%; 95% confidence interval [CI], 0.01 to 1.7) in the levonorgestrel group and 0 in 321 (0%; 95% CI, 0 to 1.1) in the copper IUD group; the between-group absolute difference in both analyses was 0.3 percentage points (95% CI, -0.9 to 1.8), consistent with the noninferiority of the levonorgestrel IUD to the copper IUD. Adverse events resulting in participants seeking medical care in the first month after IUD placement occurred in 5.2% of participants in the levonorgestrel IUD group and 4.9% of those in the copper IUD group.
Conclusions: The levonorgestrel IUD was noninferior to the copper IUD for emergency contraception. (Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and others; ClinicalTrials.gov number, NCT02175030.).
Copyright © 2021 Massachusetts Medical Society.
Figures
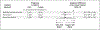
References
-
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375: 555–62. - PubMed
-
- von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet 2002; 360: 1803–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
