Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study
- PMID: 33503393
- PMCID: PMC8078423
- DOI: 10.1200/JCO.20.02088
Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study
Abstract
Purpose: BEACON CRC evaluated encorafenib plus cetuximab with or without binimetinib versus investigators' choice of irinotecan or FOLFIRI plus cetuximab in patients with BRAFV600E-mutant metastatic colorectal cancer (mCRC), after progression on 1-2 prior regimens. In the previously reported primary analysis, encorafenib, binimetinib plus cetuximab (ENCO/BINI/CETUX; triplet) and encorafenib plus cetuximab (ENCO/CETUX; doublet) regimens improved overall survival (OS) and objective response rate (ORR; by blinded central review) versus standard of care. The purpose of this analysis was to report updated efficacy and safety data.
Methods: In this open-label, phase III trial, 665 patients with BRAF V600E-mutant mCRC were randomly assigned 1:1:1 to receive triplet, doublet, or control. Primary end points were OS and independently reviewed ORR comparing triplet to control. OS for doublet versus control was a key secondary end point. Updated analyses include 6 months of additional follow-up and ORR for all randomized patients.
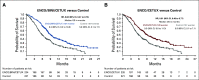
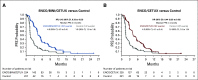
Results: Patients received triplet (n = 224), doublet (n = 220), or control (n = 221). Median OS was 9.3 months (95% CI, 8.2 to 10.8) for triplet and 5.9 months (95% CI, 5.1 to 7.1) for control (hazard ratio [HR], 0.60 [95% CI, 0.47 to 0.75]). Median OS for doublet was 9.3 months (95% CI, 8.0 to 11.3) (HR v control, 0.61 [95% CI, 0.48 to 0.77]). Confirmed ORR was 26.8% (95% CI, 21.1% to 33.1%) for triplet, 19.5% (95% CI, 14.5% to 25.4%) for doublet, and 1.8% (95% CI, 0.5% to 4.6%) for control. Adverse events were consistent with the prior primary analysis, with grade ≥ 3 adverse events in 65.8%, 57.4%, and 64.2% for triplet, doublet, and control, respectively.
Conclusion: In the BEACON CRC study, encorafenib plus cetuximab improved OS, ORR, and progression-free survival in previously treated patients in the metastatic setting compared with standard chemotherapy. Based on the primary and updated analyses, encorafenib plus cetuximab is a new standard care regimen for previously treated patients with BRAF V600E mCRC.
Trial registration: ClinicalTrials.gov NCT02928224.
Figures

Comment in
-
BRAF Mutation in Colorectal Cancer: An Enigmatic Target.J Clin Oncol. 2021 Feb 1;39(4):259-261. doi: 10.1200/JCO.20.03043. J Clin Oncol. 2021. PMID: 33503394 No abstract available.
References
-
- Davies H Bignell GR Cox C, et al. : Mutations of the BRAF gene in human cancer. Nature 417:949-954, 2002 - PubMed
-
- Kayhanian H Goode E Sclafani F, et al. : Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: A retrospective matched case-control study. Clin Colorectal Cancer 17:e69-e76, 2018 - PubMed
-
- De Roock W Claes B Bernasconi D, et al. : Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol 11:753-762, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

