Proton-activated chloride channel PAC regulates endosomal acidification and transferrin receptor-mediated endocytosis
- PMID: 33503418
- PMCID: PMC7869721
- DOI: 10.1016/j.celrep.2020.108683
Proton-activated chloride channel PAC regulates endosomal acidification and transferrin receptor-mediated endocytosis
Abstract
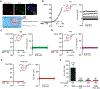
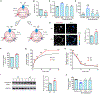
During vesicular acidification, chloride (Cl-), as the counterion, provides the electrical shunt for proton pumping by the vacuolar H+ ATPase. Intracellular CLC transporters mediate Cl- influx to the endolysosomes through their 2Cl-/H+ exchange activity. However, whole-endolysosomal patch-clamp recording also revealed a mysterious conductance releasing Cl- from the lumen. It remains unknown whether CLCs or other Cl- channels are responsible for this activity. Here, we show that the newly identified proton-activated Cl- (PAC) channel traffics from the plasma membrane to endosomes via the classical YxxL motif. PAC deletion abolishes the endosomal Cl- conductance, raises luminal Cl- level, lowers luminal pH, and increases transferrin receptor-mediated endocytosis. PAC overexpression generates a large endosomal Cl- current with properties similar to those of endogenous conductance, hypo-acidifies endosomal pH, and reduces transferrin uptake. We propose that the endosomal Cl- PAC channel functions as a low pH sensor and prevents hyper-acidification by releasing Cl- from the lumen.
Keywords: ASOR; PACC1; PAORAC; TMEM206; hPAC; organellar ion channel.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

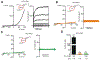
References
-
- Bertl A, Blumwald E, Coronado R, Eisenberg R, Findlay G, Gradmann D, Hille B, Köhler K, Kolb HA, MacRobbie E, et al. (1992). Electrical measurements on endomembranes. Science 258, 873–874. - PubMed
-
- Cang C, Bekele B, and Ren D (2014). The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol 10, 463–469. - PubMed
-
- Cang C, Aranda K, Seo YJ, Gasnier B, and Ren D (2015). TMEM175 Is an Organelle K(+) Channel Regulating Lysosomal Function. Cell 162, 1101–1112. - PubMed
-
- Capurro V, Gianotti A, Caci E, Ravazzolo R, Galietta LJ, and Zegarra-Moran O (2015). Functional analysis of acid-activated Cl− channels: properties and mechanisms of regulation. Biochim. Biophys. Acta 1848 (1 Pt A), 105–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

