Gut feelings about bacterial steroid-17,20-desmolase
- PMID: 33503463
- PMCID: PMC8886824
- DOI: 10.1016/j.mce.2021.111174
Gut feelings about bacterial steroid-17,20-desmolase
Abstract
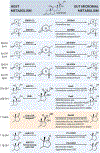
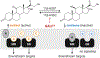
Advances in technology are only beginning to reveal the complex interactions between hosts and their resident microbiota that have co-evolved over centuries. In this review, we present compelling evidence that implicates the host-associated microbiome in the generation of 11β-hydroxyandrostenedione, leading to the formation of potent 11-oxy-androgens. Microbial steroid-17,20-desmolase cleaves the side-chain of glucocorticoids (GC), including cortisol (and its derivatives of cortisone, 5α-dihydrocortisol, and also (allo)- 3α, 5α-tetrahydrocortisol, but not 3α-5β-tetrahydrocortisol) and drugs (prednisone and dexamethasone). In addition to side-chain cleavage, we discuss the gut microbiome's robust potential to transform a myriad of steroids, mirroring much of the host's metabolism. We also explore the overlooked role of intestinal steroidogenesis and efflux pumps as a potential route for GC transport into the gut. Lastly, we propose several health implications from microbial steroid-17,20-desmolase function, including aberrant mineralocorticoid, GC, and androgen receptor signaling in colonocytes, immune cells, and prostate cells, which may exacerbate disease states.
Keywords: 11-Oxy-androgen; Cortisol; GALF; Glucocorticoid; Hydroxysteroid dehydrogenase; Microbiome; Steroid-17,20-desmolase; Sterolbiome.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Bacterial steroid-17,20-desmolase is a taxonomically rare enzymatic pathway that converts prednisone to 1,4-androstanediene-3,11,17-trione, a metabolite that causes proliferation of prostate cancer cells.J Steroid Biochem Mol Biol. 2020 May;199:105567. doi: 10.1016/j.jsbmb.2019.105567. Epub 2019 Dec 20. J Steroid Biochem Mol Biol. 2020. PMID: 31870912 Free PMC article.
-
Structural and biochemical characterization of 20β-hydroxysteroid dehydrogenase from Bifidobacterium adolescentis strain L2-32.J Biol Chem. 2019 Aug 9;294(32):12040-12053. doi: 10.1074/jbc.RA119.009390. Epub 2019 Jun 17. J Biol Chem. 2019. PMID: 31209107 Free PMC article.
-
CYP17A1 exhibits 17αhydroxylase/17,20-lyase activity towards 11β-hydroxyprogesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway.J Steroid Biochem Mol Biol. 2020 May;199:105614. doi: 10.1016/j.jsbmb.2020.105614. Epub 2020 Jan 30. J Steroid Biochem Mol Biol. 2020. PMID: 32007561
-
An alternative explanation of hypertension associated with 17α-hydroxylase deficiency syndrome.Steroids. 2014 Jan;79:44-8. doi: 10.1016/j.steroids.2013.10.006. Epub 2013 Oct 28. Steroids. 2014. PMID: 24176792 Review.
-
Glucocorticoids and gut bacteria: "The GALF Hypothesis" in the metagenomic era.Steroids. 2017 Sep;125:1-13. doi: 10.1016/j.steroids.2017.06.002. Epub 2017 Jun 15. Steroids. 2017. PMID: 28624548 Review.
Cited by
-
Genome-wide analysis revealed the stepwise origin and functional diversification of HSDs from lower to higher plant species.Front Plant Sci. 2023 Jun 15;14:1159394. doi: 10.3389/fpls.2023.1159394. eCollection 2023. Front Plant Sci. 2023. PMID: 37396629 Free PMC article.
-
Microbial metabolism marvels: a comprehensive review of microbial drug transformation capabilities.Gut Microbes. 2024 Jan-Dec;16(1):2387400. doi: 10.1080/19490976.2024.2387400. Epub 2024 Aug 16. Gut Microbes. 2024. PMID: 39150897 Free PMC article. Review.
-
Hormonal Environment Shapes the Oral Microbiome.Adv Exp Med Biol. 2025;1472:225-242. doi: 10.1007/978-3-031-79146-8_14. Adv Exp Med Biol. 2025. PMID: 40111695 Review.
-
Clostridium scindens: history and current outlook for a keystone species in the mammalian gut involved in bile acid and steroid metabolism.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf016. doi: 10.1093/femsre/fuaf016. FEMS Microbiol Rev. 2025. PMID: 40307670 Free PMC article. Review.
-
Blockade of 11β-hydroxysteroid dehydrogenase type 1 ameliorates metabolic dysfunction-associated steatotic liver disease and fibrosis.Heliyon. 2024 Oct 18;10(20):e39534. doi: 10.1016/j.heliyon.2024.e39534. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498052 Free PMC article.
References
-
- Shackleton CHL, Neres MS, Hughes BA, Stewart PM & Kater CE 844 17-Hydroxylase/C17,20-lyase (CYP17) is not the enzyme responsible for side-chain cleavage of cortisol and its metabolites. Steroids 73, 652–656 (2008). - PubMed
-
- Shackleton CHL, Neres MS, Hughes BA, Stewart PM & Kater CE 17-Hydroxylase/C17,20-lyase (CYP17) is not the enzyme responsible for side-chain cleavage of cortisol and its metabolites. Steroids 73, 652–656 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

