Estrogen Receptor Signaling Pathways Involved in Invasion and Colony Formation of Androgen-Independent Prostate Cancer Cells PC-3
- PMID: 33503805
- PMCID: PMC7865506
- DOI: 10.3390/ijms22031153
Estrogen Receptor Signaling Pathways Involved in Invasion and Colony Formation of Androgen-Independent Prostate Cancer Cells PC-3
Abstract
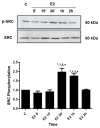
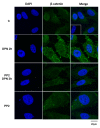
Castration-resistant prostate cancer (CRPC) is an advanced and androgen-independent form of prostate cancer. Recent studies of rapid actions mediated by estrogen in the prostate and its relationship with CRPC are emerging. We have previously shown that estrogen receptor (ER) promotes migration and invasion of the androgen-independent prostate cancer cells PC-3, but the signaling pathways involved in these events remain to be elucidated. Therefore, this study aimed to analyze the role of ERα and ERβ in the activation of SRC, and the involvement of SRC and PI3K/AKT on invasion and colony formation of the PC-3 cells. Our results showed that the activation of ERα (using ERα-selective agonist PPT) and ERβ (using ERβ-selective agonist DPN) increased phosphorylation of SRC in PC-3 cells. In the presence of the selective inhibitor for SRC-family kinases PP2, the effects of DPN and PPT on transmigration and soft agar colony formation assays were decreased. Furthermore, SRC is involved in the expression of the non-phosphorylated β-catenin. Finally, using PI3K specific inhibitor Wortmannin and AKT inhibitor MK2206, we showed that PI3K/AKT are also required for invasion and colony formation of PC-3 cells simulated by ER. This study provides novel insights into molecular mechanisms of ER in PC-3 cells by demonstrating that ER, located outside the cell nucleus, activates rapid responses molecules, including SRC and PI3K/AKT, which enhance the tumorigenic potential of prostate cancer cells, increasing cell proliferation, migration, invasion, and tumor formation.
Keywords: PC-3 cells; PI3K/AKT; SRC; estrogen receptor; β-catenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Estrogen receptor beta (ERβ) mediates expression of β-catenin and proliferation in prostate cancer cell line PC-3.Mol Cell Endocrinol. 2016 Jul 15;430:12-24. doi: 10.1016/j.mce.2016.04.012. Epub 2016 Apr 21. Mol Cell Endocrinol. 2016. PMID: 27107935
-
Estrogen Receptors Promote Migration, Invasion and Colony Formation of the Androgen-Independent Prostate Cancer Cells PC-3 Through β-Catenin Pathway.Front Endocrinol (Lausanne). 2020 Apr 9;11:184. doi: 10.3389/fendo.2020.00184. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32328032 Free PMC article.
-
Activation of estrogen receptor beta (ERβ) regulates the expression of N-cadherin, E-cadherin and β-catenin in androgen-independent prostate cancer cells.Int J Biochem Cell Biol. 2018 Mar;96:40-50. doi: 10.1016/j.biocel.2018.01.008. Epub 2018 Jan 16. Int J Biochem Cell Biol. 2018. PMID: 29341930
-
Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression.Prostate. 2018 Jan;78(1):2-10. doi: 10.1002/pros.23446. Epub 2017 Nov 2. Prostate. 2018. PMID: 29094395 Review.
-
Comparison of the roles of estrogens and androgens in breast cancer and prostate cancer.J Cell Biochem. 2020 Apr;121(4):2756-2769. doi: 10.1002/jcb.29515. Epub 2019 Nov 6. J Cell Biochem. 2020. PMID: 31693255 Review.
Cited by
-
The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens.Biomedicines. 2024 Jul 23;12(8):1636. doi: 10.3390/biomedicines12081636. Biomedicines. 2024. PMID: 39200101 Free PMC article. Review.
-
Selective estrogen receptor modulators contribute to prostate cancer treatment by regulating the tumor immune microenvironment.J Immunother Cancer. 2022 Apr;10(4):e002944. doi: 10.1136/jitc-2021-002944. J Immunother Cancer. 2022. PMID: 35383112 Free PMC article. Review.
-
The Role of ERα and ERβ in Castration-Resistant Prostate Cancer and Current Therapeutic Approaches.Biomedicines. 2023 Mar 9;11(3):826. doi: 10.3390/biomedicines11030826. Biomedicines. 2023. PMID: 36979805 Free PMC article. Review.
-
Transcriptomic Profile of Perineural Invasion in Prostate Cancer Identifies Prognostic Gene Signatures.Biomedicines. 2025 Jul 22;13(8):1789. doi: 10.3390/biomedicines13081789. Biomedicines. 2025. PMID: 40868045 Free PMC article.
-
Involvement of Sex Hormones and Their Receptors in the Pathogenesis of Classic Kaposi's Sarcoma in Xinjiang.Skin Res Technol. 2024 Oct;30(10):e70086. doi: 10.1111/srt.70086. Skin Res Technol. 2024. PMID: 39349354 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

