JAK2 Inhibition by Fedratinib Reduces Osteoblast Differentiation and Mineralisation of Human Mesenchymal Stem Cells
- PMID: 33503825
- PMCID: PMC7866227
- DOI: 10.3390/molecules26030606
JAK2 Inhibition by Fedratinib Reduces Osteoblast Differentiation and Mineralisation of Human Mesenchymal Stem Cells
Abstract
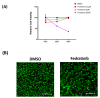
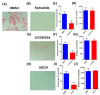
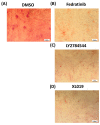
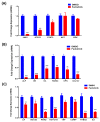
Several signalling pathways, including the JAK/STAT signalling pathway, have been identified to regulate the differentiation of human bone marrow skeletal (mesenchymal) stem cells (hBMSCs) into bone-forming osteoblasts. Members of the JAK family mediate the intracellular signalling of various of cytokines and growth factors, leading to the regulation of cell proliferation and differentiation into bone-forming osteoblastic cells. Inhibition of JAK2 leads to decoupling of its downstream mediator, STAT3, and the subsequent inhibition of JAK/STAT signalling. However, the crucial role of JAK2 in hBMSCs biology has not been studied in detail. A JAK2 inhibitor, Fedratinib, was identified during a chemical biology screen of a small molecule library for effects on the osteoblastic differentiation of hMSC-TERT cells. Alkaline phosphatase activity and staining assays were conducted as indicators of osteoblastic differentiation, while Alizarin red staining was used as an indicator of in vitro mineralised matrix formation. Changes in gene expression were assessed using quantitative real-time polymerase chain reaction. Fedratinib exerted significant inhibitory effects on the osteoblastic differentiation of hMSC-TERT cells, as demonstrated by reduced ALP activity, in vitro mineralised matrix formation and downregulation of osteoblast-related gene expression, including ALP, ON, OC, RUNX2, OPN, and COL1A1. To identify the underlying molecular mechanisms, we examined the effects of Fedratinib on a molecular signature of several target genes known to affect hMSC-TERT differentiation into osteoblasts. Fedratinib inhibited the expression of LIF, SOCS3, RRAD, NOTCH3, TNF, COMP, THBS2, and IL6, which are associated with various signalling pathways, including TGFβ signalling, insulin signalling, focal adhesion, Notch Signalling, IL-6 signalling, endochondral ossification, TNF-α, and cytokines and inflammatory response. We identified a JAK2 inhibitor (Fedratinib) as a powerful inhibitor of the osteoblastic differentiation of hMSC-TERT cells, which may be useful as a therapeutic option for treating conditions associated with ectopic bone formation or osteosclerotic metastases.
Keywords: Fedratinib; JAK/STAT signalling pathway; JAK2 inhibition; hMSC-TERT; in vitro mineralisation; osteoblast differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Adam S., Simon N., Steffen U., Andes F.T., Scholtysek C., Muller D.I.H., Weidner D., Andreev D., Kleyer A., Culemann S., et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aay4447. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

