Mycoplasma pneumoniae Infections: Pathogenesis and Vaccine Development
- PMID: 33503845
- PMCID: PMC7911756
- DOI: 10.3390/pathogens10020119
Mycoplasma pneumoniae Infections: Pathogenesis and Vaccine Development
Abstract
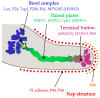
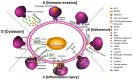
Mycoplasma pneumoniae is a major causative agent of community-acquired pneumonia which can lead to both acute upper and lower respiratory tract inflammation, and extrapulmonary syndromes. Refractory pneumonia caused by M. pneumonia can be life-threatening, especially in infants and the elderly. Here, based on a comprehensive review of the scientific literature related to the respective area, we summarize the virulence factors of M. pneumoniae and the major pathogenic mechanisms mediated by the pathogen: adhesion to host cells, direct cytotoxicity against host cells, inflammatory response-induced immune injury, and immune evasion. The increasing rate of macrolide-resistant strains and the harmful side effects of other sensitive antibiotics (e.g., respiratory quinolones and tetracyclines) in young children make it difficult to treat, and increase the health risk or re-infections. Hence, there is an urgent need for development of an effective vaccine to prevent M. pneumoniae infections in children. Various types of M. pneumoniae vaccines have been reported, including whole-cell vaccines (inactivated and live-attenuated vaccines), subunit vaccines (involving M. pneumoniae protein P1, protein P30, protein P116 and CARDS toxin) and DNA vaccines. This narrative review summarizes the key pathogenic mechanisms underlying M. pneumoniae infection and highlights the relevant vaccines that have been developed and their reported effectiveness.
Keywords: DNA vaccines; Mycoplasma pneumonia; live vector vaccines; pathogenesis; subunit vaccines; virulence factors; whole-cell vaccine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Xu W., Guo L., Dong X., Li X., Zhou P., Ni Q., Zhou X.Y., Wagner B.L., Li L. Detection of viruses and Mycoplasma pneumoniae in hospitalized patients with severe acute respiratory infection in northern China, 2015–2016. Jpn. J. Infect. Dis. 2018;71:134–139. doi: 10.7883/yoken.JJID.2017.412. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

