The Roles of Inflammasomes in Host Defense against Mycobacterium tuberculosis
- PMID: 33503864
- PMCID: PMC7911501
- DOI: 10.3390/pathogens10020120
The Roles of Inflammasomes in Host Defense against Mycobacterium tuberculosis
Abstract
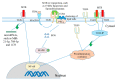
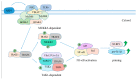
Mycobacterium tuberculosis (MTB) infection is characterized by granulomatous lung lesions and systemic inflammatory responses during active disease. Inflammasome activation is involved in regulation of inflammation. Inflammasomes are multiprotein complexes serving a platform for activation of caspase-1, which cleaves the proinflammatory cytokines such as interleukin-1β (IL-1β) and IL-18 into their active forms. These cytokines play an essential role in MTB control. MTB infection triggers activation of the nucleotide-binding domain, leucine-rich-repeat containing family, pyrin domain-containing 3 (NLRP3) and absent in melanoma 2 (AIM2) inflammasomes in vitro, but only AIM2 and apoptosis-associated speck-like protein containing a caspase-activation recruitment domain (ASC), rather than NLRP3 or caspase-1, favor host survival and restriction of mycobacterial replication in vivo. Interferons (IFNs) inhibits MTB-induced inflammasome activation and IL-1 signaling. In this review, we focus on activation and regulation of the NLRP3 and AIM2 inflammasomes after exposure to MTB, as well as the effect of inflammasome activation on host defense against the infection.
Keywords: AIM2; IFN; Mycobacterium tuberculosis; NLRP3; inflammasome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Herpes simplex virus type 1 inflammasome activation in proinflammatory human macrophages is dependent on NLRP3, ASC, and caspase-1.PLoS One. 2020 Feb 26;15(2):e0229570. doi: 10.1371/journal.pone.0229570. eCollection 2020. PLoS One. 2020. PMID: 32101570 Free PMC article.
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
-
Mycobacterium tuberculosis and the host cell inflammasome: a complex relationship.Front Cell Infect Microbiol. 2013 Oct 9;3:62. doi: 10.3389/fcimb.2013.00062. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24130966 Free PMC article. Review.
Cited by
-
Systematic review of innate immune responses against Mycobacterium tuberculosis complex infection in animal models.Front Immunol. 2025 Jan 30;15:1467016. doi: 10.3389/fimmu.2024.1467016. eCollection 2024. Front Immunol. 2025. PMID: 39949719 Free PMC article.
-
Crosstalk between Interleukin-1β and Type I Interferons Signaling in Autoinflammatory Diseases.Cells. 2021 May 8;10(5):1134. doi: 10.3390/cells10051134. Cells. 2021. PMID: 34066649 Free PMC article. Review.
-
PGC-1α activation to enhance macrophage immune function in mycobacterial infections.PLoS One. 2025 Feb 6;20(2):e0310908. doi: 10.1371/journal.pone.0310908. eCollection 2025. PLoS One. 2025. PMID: 39913377 Free PMC article.
-
Mycobacterium tuberculosis PE_PGRS38 Enhances Intracellular Survival of Mycobacteria by Inhibiting TLR4/NF-κB-Dependent Inflammation and Apoptosis of the Host.Biology (Basel). 2024 Apr 30;13(5):313. doi: 10.3390/biology13050313. Biology (Basel). 2024. PMID: 38785795 Free PMC article.
-
Fluvastatin Converts Human Macrophages into Foam Cells with Increased Inflammatory Response to Inactivated Mycobacterium tuberculosis H37Ra.Cells. 2024 Mar 18;13(6):536. doi: 10.3390/cells13060536. Cells. 2024. PMID: 38534380 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

