Ventricular Assist Device-Specific Infections
- PMID: 33503891
- PMCID: PMC7866069
- DOI: 10.3390/jcm10030453
Ventricular Assist Device-Specific Infections
Abstract
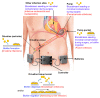
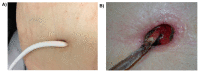
Ventricular assist device (VAD)-specific infections, in particular, driveline infections, are a concerning complication of VAD implantation that often results in significant morbidity and even mortality. The presence of a percutaneous driveline at the skin exit-site and in the subcutaneous tunnel allows biofilm formation and migration by many bacterial and fungal pathogens. Biofilm formation is an important microbial strategy, providing a shield against antimicrobial treatment and human immune responses; biofilm migration facilitates the extension of infection to deeper tissues such as the pump pocket and the bloodstream. Despite the introduction of multiple preventative strategies, driveline infections still occur with a high prevalence of ~10-20% per year and their treatment outcomes are frequently unsatisfactory. Clinical diagnosis, prevention and management of driveline infections are being targeted to specific microbial pathogens grown as biofilms at the driveline exit-site or in the driveline tunnel. The purpose of this review is to improve the understanding of VAD-specific infections, from basic "bench" knowledge to clinical "bedside" experience, with a specific focus on the role of biofilms in driveline infections.
Keywords: biofilms; driveline infections; driveline tunnel; epidemiology; exit-site; prevention; treatment; ventricular assist device.
Conflict of interest statement
D.M. is an Abbott proctor for implantation of the Heartmate III VAD. Y.Q., A.Y.P., and D.M. received an external research program grant from Medtronic. Neither Abbott or Medtronic played a role in the writing of this manuscript.
Figures



Similar articles
-
Biofilm formation and migration on ventricular assist device drivelines.J Thorac Cardiovasc Surg. 2020 Feb;159(2):491-502.e2. doi: 10.1016/j.jtcvs.2019.02.088. Epub 2019 Mar 6. J Thorac Cardiovasc Surg. 2020. PMID: 30955967
-
Characterization of infected, explanted ventricular assist device drivelines: The role of biofilms and microgaps in the driveline tunnel.J Heart Lung Transplant. 2020 Nov;39(11):1289-1299. doi: 10.1016/j.healun.2020.07.015. Epub 2020 Jul 24. J Heart Lung Transplant. 2020. PMID: 32771438
-
In vitro Evaluation of Medihoney Antibacterial Wound Gel as an Anti-biofilm Agent Against Ventricular Assist Device Driveline Infections.Front Microbiol. 2020 Nov 23;11:605608. doi: 10.3389/fmicb.2020.605608. eCollection 2020. Front Microbiol. 2020. PMID: 33329497 Free PMC article.
-
Ventricular Assist Device Driveline Infections: A Systematic Review.Thorac Cardiovasc Surg. 2022 Sep;70(6):493-504. doi: 10.1055/s-0041-1731823. Epub 2021 Sep 14. Thorac Cardiovasc Surg. 2022. PMID: 34521143
-
Prevention and early treatment of driveline infections in ventricular assist device patients - The DESTINE staging proposal and the first standard of care protocol.J Crit Care. 2020 Apr;56:106-112. doi: 10.1016/j.jcrc.2019.12.014. Epub 2019 Dec 17. J Crit Care. 2020. PMID: 31896443 Review.
Cited by
-
Left ventricular assist device-associated driveline infections as a specific form of complicated skin and soft tissue infection/acute bacterial skin and skin structure infection - issues and therapeutic options.Curr Opin Infect Dis. 2024 Apr 1;37(2):95-104. doi: 10.1097/QCO.0000000000000999. Epub 2024 Jan 15. Curr Opin Infect Dis. 2024. PMID: 38085707 Free PMC article. Review.
-
Anti-infective characteristics of a new Carbothane ventricular assist device driveline.Biofilm. 2023 Apr 17;5:100124. doi: 10.1016/j.bioflm.2023.100124. eCollection 2023 Dec. Biofilm. 2023. PMID: 37153749 Free PMC article.
-
Survival of a patient following initial left ventricular assist device implantation and two successive left ventricular assist device exchanges: case report.Eur Heart J Case Rep. 2024 Nov 16;8(12):ytae618. doi: 10.1093/ehjcr/ytae618. eCollection 2024 Dec. Eur Heart J Case Rep. 2024. PMID: 39669551 Free PMC article.
-
Systems of conductive skin for power transfer in clinical applications.Eur Biophys J. 2022 Mar;51(2):171-184. doi: 10.1007/s00249-021-01568-8. Epub 2021 Sep 3. Eur Biophys J. 2022. PMID: 34477935 Free PMC article. Review.
-
Driveline Features as Risk Factor for Infection in Left Ventricular Assist Devices: Meta-Analysis and Experimental Tests.Front Cardiovasc Med. 2021 Dec 16;8:784208. doi: 10.3389/fcvm.2021.784208. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34977190 Free PMC article.
References
-
- Marasco S.F., McDonald C., McGiffin D.C. Surgical implantation. In: Gregory S.D., Stevens M.C., Fraser J.F., editors. Mechanical Circulatory and Respiratory Support. Academic Press; Cambridge, CA, USA: 2018.
-
- Zierer A., Melby S.J., Voeller R.K., Guthrie T.J., Ewald G.A., Shelton K., Pasque M.K., Moon M.R., Damiano R.J., Jr., Moazami N. Late-onset driveline infections: The Achilles’ heel of prolonged left ventricular assist device support. Ann. Thorac. Surg. 2007;84:515–520. doi: 10.1016/j.athoracsur.2007.03.085. - DOI - PubMed
-
- De By T., Mohacsi P., Gahl B., Zittermann A., Krabatsch T., Gustafsson F., Leprince P., Meyns B., Netuka I., Caliskan K., et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): Second report. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2018;53:309–316. doi: 10.1093/ejcts/ezx320. - DOI - PubMed
-
- Kormos R.L., Cowger J., Pagani F.D., Teuteberg J.J., Goldstein D.J., Jacobs J.P., Higgins R.S., Stevenson L.W., Stehlik J., Atluri P., et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving indications, outcomes, and scientific partnerships. Ann. Thorac. Surg. 2019;107:341–353. doi: 10.1016/j.athoracsur.2018.11.011. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

