Ibudilast Mitigates Delayed Bone Healing Caused by Lipopolysaccharide by Altering Osteoblast and Osteoclast Activity
- PMID: 33503906
- PMCID: PMC7865869
- DOI: 10.3390/ijms22031169
Ibudilast Mitigates Delayed Bone Healing Caused by Lipopolysaccharide by Altering Osteoblast and Osteoclast Activity
Abstract
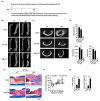
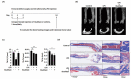
Bacterial infection in orthopedic surgery is challenging because cell wall components released after bactericidal treatment can alter osteoblast and osteoclast activity and impair fracture stability. However, the precise effects and mechanisms whereby cell wall components impair bone healing are unclear. In this study, we characterized the effects of lipopolysaccharide (LPS) on bone healing and osteoclast and osteoblast activity in vitro and in vivo and evaluated the effects of ibudilast, an antagonist of toll-like receptor 4 (TLR4), on LPS-induced changes. In particular, micro-computed tomography was used to reconstruct femoral morphology and analyze callus bone content in a femoral defect mouse model. In the sham-treated group, significant bone bridge and cancellous bone formation were observed after surgery, however, LPS treatment delayed bone bridge and cancellous bone formation. LPS inhibited osteogenic factor-induced MC3T3-E1 cell differentiation, alkaline phosphatase (ALP) levels, calcium deposition, and osteopontin secretion and increased the activity of osteoclast-associated molecules, including cathepsin K and tartrate-resistant acid phosphatase in vitro. Finally, ibudilast blocked the LPS-induced inhibition of osteoblast activation and activation of osteoclast in vitro and attenuated LPS-induced delayed callus bone formation in vivo. Our results provide a basis for the development of a novel strategy for the treatment of bone infection.
Keywords: bone bridge; bone healing; callus bone; femoral defect; ibudilast; lipopolysaccharide; osteoblast; osteoclast.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Rochford E.T., Sabate Bresco M., Zeiter S., Kluge K., Poulsson A., Ziegler M., Richards R.G., O’Mahony L., Moriarty T.F. Monitoring immune responses in a mouse model of fracture fixation with and without Staphylococcus aureus osteomyelitis. Bone. 2016;83:82–92. doi: 10.1016/j.bone.2015.10.014. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

