Deciphering the Disaggregation Mechanism of Amyloid Beta Aggregate by 4-(2-Hydroxyethyl)-1-Piperazinepropanesulfonic Acid Using Electrochemical Impedance Spectroscopy
- PMID: 33503934
- PMCID: PMC7865397
- DOI: 10.3390/s21030788
Deciphering the Disaggregation Mechanism of Amyloid Beta Aggregate by 4-(2-Hydroxyethyl)-1-Piperazinepropanesulfonic Acid Using Electrochemical Impedance Spectroscopy
Abstract
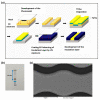
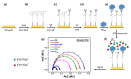
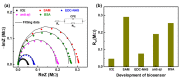
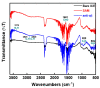
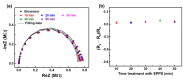
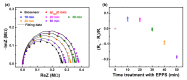
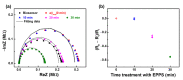
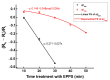
Aggregation of amyloid-β (aβ) peptides into toxic oligomers, fibrils, and plaques is central in the molecular pathogenesis of Alzheimer's disease (AD) and is the primary focus of AD diagnostics. Disaggregation or elimination of toxic aβ aggregates in patients is important for delaying the progression of neurodegenerative disorders in AD. Recently, 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid (EPPS) was introduced as a chemical agent that binds with toxic aβ aggregates and transforms them into monomers to reduce the negative effects of aβ aggregates in the brain. However, the mechanism of aβ disaggregation by EPPS has not yet been completely clarified. In this study, an electrochemical impedimetric immunosensor for aβ diagnostics was developed by immobilizing a specific anti-amyloid-β (aβ) antibody onto a self-assembled monolayer functionalized with a new interdigitated chain-shaped electrode (anti-aβ/SAM/ICE). To investigate the ability of EPPS in recognizing AD by extricating aβ aggregation, commercially available aβ aggregates (aβagg) were used. Electrochemical impedance spectroscopy was used to probe the changes in charge transfer resistance (Rct) of the immunosensor after the specific binding of biosensor with aβagg. The subsequent incubation of the aβagg complex with a specific concentration of EPPS at different time intervals divulged AD progression. The decline in the Rct of the immunosensor started at 10 min of EPPS incubation and continued to decrease gradually from 20 min, indicating that the accumulation of aβagg on the surface of the anti-aβ/SAM/ICE sensor has been extricated. Here, the kinetic disaggregation rate k value of aβagg was found to be 0.038. This innovative study using electrochemical measurement to investigate the mechanism of aβagg disaggregation by EPPS could provide a new perspective in monitoring the disaggregation periods of aβagg from oligomeric to monomeric form, and then support for the prediction and handling AD symptoms at different stages after treatment by a drug, EPPS.
Keywords: 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid; Alzheimer’s disease; amyloid beta aggregate; electrochemical impedance spectroscopy; impedimetric immunosensor; kinetic disaggregation of protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Atomic Force Microscopy Analysis of EPPS-Driven Degradation and Reformation of Amyloid-β Aggregates.J Alzheimers Dis Rep. 2018 Feb 16;2(1):41-49. doi: 10.3233/ADR-170024. J Alzheimers Dis Rep. 2018. PMID: 30480247 Free PMC article.
-
Chemical-Driven Amyloid Clearance for Therapeutics and Diagnostics of Alzheimer's Disease.Acc Chem Res. 2024 Nov 19;57(22):3266-3276. doi: 10.1021/acs.accounts.4c00458. Epub 2024 Nov 4. Acc Chem Res. 2024. PMID: 39496112
-
Sensitive electrochemical detection of amyloid beta peptide in human serum using an interdigitated chain-shaped electrode.Biosens Bioelectron. 2019 Nov 1;144:111694. doi: 10.1016/j.bios.2019.111694. Epub 2019 Sep 7. Biosens Bioelectron. 2019. PMID: 31539720
-
Electrochemistry of Alzheimer Disease Amyloid Beta Peptides.Curr Med Chem. 2018;25(33):4066-4083. doi: 10.2174/0929867325666180214112536. Curr Med Chem. 2018. PMID: 29446720 Review.
-
Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer's disease.Acta Neuropathol. 2015 Feb;129(2):167-82. doi: 10.1007/s00401-014-1375-y. Epub 2014 Dec 23. Acta Neuropathol. 2015. PMID: 25534025 Review.
Cited by
-
A Microfluidic Platform with an Embedded Miniaturized Electrochemical Sensor for On-Chip Plasma Extraction Followed by In Situ High-Sensitivity C-Reactive Protein (hs-CRP) Detection.Biosensors (Basel). 2022 Dec 13;12(12):1163. doi: 10.3390/bios12121163. Biosensors (Basel). 2022. PMID: 36551130 Free PMC article.
-
Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode.Biomedicines. 2021 Dec 22;10(1):10. doi: 10.3390/biomedicines10010010. Biomedicines. 2021. PMID: 35052691 Free PMC article.
-
Removal of Thiol-SAM on a Gold Surface for Re-Use of an Interdigitated Chain-Shaped Electrode.Materials (Basel). 2022 Mar 17;15(6):2218. doi: 10.3390/ma15062218. Materials (Basel). 2022. PMID: 35329670 Free PMC article.
-
A Multi-Chamber Paper-Based Platform for the Detection of Amyloid β Oligomers 42 via Copper-Enhanced Gold Immunoblotting.Biomolecules. 2021 Jun 26;11(7):948. doi: 10.3390/biom11070948. Biomolecules. 2021. PMID: 34206715 Free PMC article.
-
Dual Inhibitors of Amyloid-β and Tau Aggregation with Amyloid-β Disaggregating Properties: Extended In Cellulo, In Silico, and Kinetic Studies of Multifunctional Anti-Alzheimer's Agents.ACS Chem Neurosci. 2021 Jun 2;12(11):2057-2068. doi: 10.1021/acschemneuro.1c00235. Epub 2021 May 21. ACS Chem Neurosci. 2021. PMID: 34019757 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

