A Novel Salt Inducible Kinase 2 Inhibitor, ARN-3261, Sensitizes Ovarian Cancer Cell Lines and Xenografts to Carboplatin
- PMID: 33503955
- PMCID: PMC7865895
- DOI: 10.3390/cancers13030446
A Novel Salt Inducible Kinase 2 Inhibitor, ARN-3261, Sensitizes Ovarian Cancer Cell Lines and Xenografts to Carboplatin
Abstract
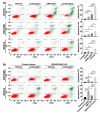
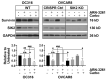
Salt-induced kinase 2 (SIK2) is a serine-threonine kinase that regulates centrosome splitting, activation of PI3 kinase and phosphorylation of class IIa HDACs, affecting gene expression. Previously, we found that inhibition of SIK2 enhanced sensitivity of ovarian cancer cells to paclitaxel. Carboplatin and paclitaxel constitute first-line therapy for most patients with ovarian carcinoma, producing a 70% clinical response rate, but curing <20% of patients with advanced disease. We have asked whether inhibition of SIK2 with ARN-3261 enhances sensitivity to carboplatin in ovarian cancer cell lines and xenograft models. ARN-3261-induced DNA damage and apoptosis were measured with γ-H2AX accumulation, comet assays, and annexin V. ARN-3261 inhibited growth of eight ovarian cancer cell lines at an IC50 of 0.8 to 3.5 µM. ARN-3261 significantly enhanced sensitivity to carboplatin in seven of eight ovarian cancer cell lines and a carboplatin-resistant cell line tested. Furthermore, ARN-3261 in combination with carboplatin produced greater inhibition of tumor growth than carboplatin alone in SKOv3 and OVCAR8 ovarian cancer xenograft models. ARN-3261 enhanced DNA damage and apoptosis by downregulating expression of survivin. Thus, a SIK2 kinase inhibitor enhanced carboplatin-induced therapy in preclinical models of ovarian cancer and deserves further evaluation in clinical trials.
Keywords: PARP inhibitor; apoptosis; carboplatin sensitivity; comet assay; salt inducible kinase 2 (SIK2); γ-H2AX.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




References
-
- Ahmed A.A., Lu Z., Jennings N.B., Etemadmoghadam D., Capalbo L., Jacamo R.O., Barbosa-Morais N., Le X.F., Australian Ovarian Cancer Study Group. Vivas-Mejia P., et al. SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell. 2010;18:109–121. doi: 10.1016/j.ccr.2010.06.018. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

