A Mathematical Model to Estimate Chemotherapy Concentration at the Tumor-Site and Predict Therapy Response in Colorectal Cancer Patients with Liver Metastases
- PMID: 33503971
- PMCID: PMC7866038
- DOI: 10.3390/cancers13030444
A Mathematical Model to Estimate Chemotherapy Concentration at the Tumor-Site and Predict Therapy Response in Colorectal Cancer Patients with Liver Metastases
Abstract
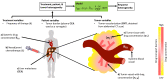
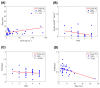
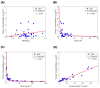
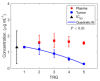
Chemotherapy remains a primary treatment for metastatic cancer, with tumor response being the benchmark outcome marker. However, therapeutic response in cancer is unpredictable due to heterogeneity in drug delivery from systemic circulation to solid tumors. In this proof-of-concept study, we evaluated chemotherapy concentration at the tumor-site and its association with therapy response by applying a mathematical model. By using pre-treatment imaging, clinical and biologic variables, and chemotherapy regimen to inform the model, we estimated tumor-site chemotherapy concentration in patients with colorectal cancer liver metastases, who received treatment prior to surgical hepatic resection with curative-intent. The differential response to therapy in resected specimens, measured with the gold-standard Tumor Regression Grade (TRG; from 1, complete response to 5, no response) was examined, relative to the model predicted systemic and tumor-site chemotherapy concentrations. We found that the average calculated plasma concentration of the cytotoxic drug was essentially equivalent across patients exhibiting different TRGs, while the estimated tumor-site chemotherapeutic concentration (eTSCC) showed a quadratic decline from TRG = 1 to TRG = 5 (p < 0.001). The eTSCC was significantly lower than the observed plasma concentration and dropped by a factor of ~5 between patients with complete response (TRG = 1) and those with no response (TRG = 5), while the plasma concentration remained stable across TRG groups. TRG variations were driven and predicted by differences in tumor perfusion and eTSCC. If confirmed in carefully planned prospective studies, these findings will form the basis of a paradigm shift in the care of patients with potentially curable colorectal cancer and liver metastases.
Keywords: FOLFOX; chemotherapy; colorectal cancer; liver metastases; mathematical model.
Conflict of interest statement
The authors do not have any conflicts of interest to declare related to this work.
Figures
References
-
- Adam R., Wicherts D.A., de Haas R.J., Aloia T., Levi F., Paule B., Guettier C., Kunstlinger F., Delvart V., Azoulay D., et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J. Clin. Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. - DOI - PubMed
-
- Blazer D.G., III, Kishi Y., Maru D.M., Kopetz S., Chun Y.S., Overman M.J., Fogelman D., Eng C., Chang D.Z., Wang H., et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. - DOI - PubMed
-
- Swisher S.G., Hofstetter W., Wu T.T., Correa A.M., Ajani J.A., Komaki R.R., Chirieac L., Hunt K.K., Liao Z., Phan A., et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT) Ann. Surg. 2005;241:810–817. doi: 10.1097/01.sla.0000161983.82345.85. - DOI - PMC - PubMed
-
- Ajani J.A., Mansfield P.F., Crane C.H., Wu T.T., Lunagomez S., Lynch P.M., Janjan N., Feig B., Faust J., Yao J.C., et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: Degree of pathologic response and not clinical parameters dictated patient outcome. J. Clin. Oncol. 2005;23:1237–1244. doi: 10.1200/JCO.2005.01.305. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

