Empagliflozin and Liraglutide Differentially Modulate Cardiac Metabolism in Diabetic Cardiomyopathy in Rats
- PMID: 33503985
- PMCID: PMC7865477
- DOI: 10.3390/ijms22031177
Empagliflozin and Liraglutide Differentially Modulate Cardiac Metabolism in Diabetic Cardiomyopathy in Rats
Abstract
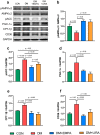
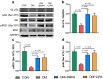
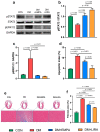
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) are antihyperglycemic agents with cardioprotective properties against diabetic cardiomyopathy (DCM). However, the distinctive mechanisms underlying GLP-1RAs and SGLT2is in DCM are not fully elucidated. The purpose of this study was to investigate the impacts of GLP1RAs and/or SGLT2is on myocardial energy metabolism, cardiac function, and apoptosis signaling in DCM. Biochemistry and echocardiograms were studied before and after treatment with empagliflozin (10 mg/kg/day, oral gavage), and/or liraglutide (200 μg/kg every 12 h, subcutaneously) for 4 weeks in male Wistar rats with streptozotocin (65 mg/kg intraperitoneally)-induced diabetes. Cardiac fibrosis, apoptosis, and protein expression of metabolic and inflammatory signaling molecules were evaluated by histopathology and Western blotting in ventricular cardiomyocytes of different groups. Empagliflozin and liraglutide normalized myocardial dysfunction in diabetic rats. Upregulation of phosphorylated-acetyl coenzyme A carboxylase, carnitine palmitoyltransferase 1β, cluster of differentiation 36, and peroxisome proliferator-activated receptor-gamma coactivator, and downregulation of glucose transporter 4, the ratio of phosphorylated adenosine monophosphate-activated protein kinase α2 to adenosine monophosphate-activated protein kinase α2, and the ratio of phosphorylated protein kinase B to protein kinase B in diabetic cardiomyocytes were restored by treatment with empagliflozin or liraglutide. Nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3, interleukin-1β, tumor necrosis factor-α, and cleaved caspase-1 were significantly downregulated in empagliflozin-treated and liraglutide-treated diabetic rats. Both empagliflozin-treated and liraglutide-treated diabetic rats exhibited attenuated myocardial fibrosis and apoptosis. Empagliflozin modulated fatty acid and glucose metabolism, while liraglutide regulated inflammation and apoptosis in DCM. The better effects of combined treatment with GLP-1RAs and SGLT2is may lead to a potential strategy targeting DCM.
Keywords: diabetic cardiomyopathy; empagliflozin; fatty acid and glucose metabolism; glucagon-like peptide 1 receptor agonists; inflammation; liraglutide; sodium-glucose cotransporter-2 inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
-
- Emerging Risk Factors C., Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. - DOI - PMC - PubMed
-
- O’Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Jr., Chung M.K., de Lemos J.A., Ettinger S.M., Fang J.C., Fesmire F.M., Franklin B.A., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742c84. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

