Immunomodulatory Properties of Mesenchymal Stromal Cells Can Vary in Genetically Modified Rats
- PMID: 33504032
- PMCID: PMC7865849
- DOI: 10.3390/ijms22031181
Immunomodulatory Properties of Mesenchymal Stromal Cells Can Vary in Genetically Modified Rats
Abstract
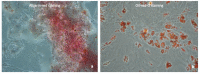
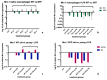
Mesenchymal Stromal Cells (MSC) have been shown to exhibit immuno-modulatory and regenerative properties at sites of inflammation. In solid organ transplantation (SOT), administration of MSCs might lead to an alleviation of ischemia-reperfusion injury and a reduction of rejection episodes. Previous reports have suggested 'MSC-preconditioning' of macrophages to be partly responsible for the beneficial effects. Whether this results from direct cell-cell interactions (e.g., MSC trans-differentiation at sites of damage), or from paracrine mechanisms, remains unclear. Immunosuppressive capacities of MSCs from donors of different age and from genetically modified donor animals, often used for in-vivo experiments, have so far not been investigated. We conducted an in vitro study to compare paracrine effects of supernatants from MSCs extracted from young and old wild-type Wystar-Kyoto rats (WKY-wt), as well as young and old WKY donor rats positive for the expression of green fluorescent protein (WKY-GFP), on bone marrow derived macrophages (BMDM). Expression levels of Mannose receptor 1 (Mrc-1), Tumor necrosis factor α (TNFα), inducible NO synthase (iNos) and Interleukin-10 (IL-10) in BMDMs after treatment with different MSC supernatants were compared by performance of quantitative PCR. We observed different expression patterns of inflammatory markers within BMDMs, depending on age and genotype of origin for MSC supernatants. This must be taken into consideration for preclinical and clinical studies, for which MSCs will be used to treat transplant patients, aiming to mitigate inflammatory and allo-responses.
Keywords: cytokines; mesenchymal stem cells; transplantation.
Conflict of interest statement
No conflicts of interest.
Figures






References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
-
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

