Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva
- PMID: 33504061
- PMCID: PMC7910854
- DOI: 10.3390/biology10020090
Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva
Abstract
The control of movements is a fundamental feature shared by all animals. At the most basic level, simple movements are generated by coordinated neural activity and muscle contraction patterns that are controlled by the central nervous system. How behavioral responses to various sensory inputs are processed and integrated by the downstream neural network to produce flexible and adaptive behaviors remains an intense area of investigation in many laboratories. Due to recent advances in experimental techniques, many fundamental neural pathways underlying animal movements have now been elucidated. For example, while the role of motor neurons in locomotion has been studied in great detail, the roles of interneurons in animal movements in both basic and noxious environments have only recently been realized. However, the genetic and transmitter identities of many of these interneurons remains unclear. In this review, we provide an overview of the underlying circuitry and neural pathways required by Drosophila larvae to produce successful movements. By improving our understanding of locomotor circuitry in model systems such as Drosophila, we will have a better understanding of how neural circuits in organisms with different bodies and brains lead to distinct locomotion types at the organism level. The understanding of genetic and physiological components of these movements types also provides directions to understand movements in higher organisms.
Keywords: Drosophila larvae; brain circuits; information processing; locomotion; neural communication; sensory systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
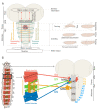
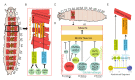
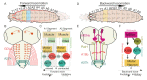
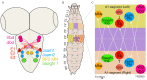
References
-
- Selverston A.I. Neural Control of Locomotion. Springer; Boston, MA, USA: 1976. Neuronal Mechanisms for Rhythmic Motor Pattern Generation in a Simple System; pp. 377–399. Advances in Behavioral Biology.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

