From Angiotensin II to Cyclic Peptides and Angiotensin Receptor Blockers (ARBs): Perspectives of ARBs in COVID-19 Therapy
- PMID: 33504092
- PMCID: PMC7865783
- DOI: 10.3390/molecules26030618
From Angiotensin II to Cyclic Peptides and Angiotensin Receptor Blockers (ARBs): Perspectives of ARBs in COVID-19 Therapy
Abstract
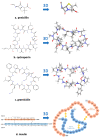
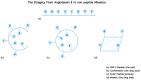
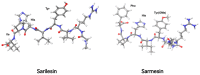
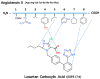
The octapeptide hormone angiotensin II is one of the most studied peptides with the aim of designing and synthesizing non-peptide mimetics for oral administration. To achieve this, cyclizations at different positions within the peptide molecule has been a useful strategy to define the active conformation. These studies on angiotensin II led to the discovery of Sarmesin, a type II angiotensin II antagonist, and the breakthrough non-peptide mimetic Losartan, the first in a series of sartans marketed as a new generation of anti-hypertensive drugs in the 1990s. Angiotensin II receptor blockers (ARBS) and angiotensin I converting enzyme inhibitors (ACEI) were recently reported to protect hypertensive patients infected with SARS-CoV-2. The renin-angiotensin system (RAS) inhibitors reduce excess angiotensin II and increase antagonist heptapeptides alamandine and aspamandine which counterbalance angiotensin II and maintain homeostasis and vasodilation.
Keywords: Covid 19; RAS; Sarmesin; Sars-CoV-2; Sartans; angiotensin II; cyclic peptides; mimetics; transdermal delivery.
Conflict of interest statement
The authors have no financial/commercial conflicts of interest to declare.
Figures






Similar articles
-
Actions of Novel Angiotensin Receptor Blocking Drugs, Bisartans, Relevant for COVID-19 Therapy: Biased Agonism at Angiotensin Receptors and the Beneficial Effects of Neprilysin in the Renin Angiotensin System.Molecules. 2022 Jul 29;27(15):4854. doi: 10.3390/molecules27154854. Molecules. 2022. PMID: 35956801 Free PMC article.
-
Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update.High Blood Press Cardiovasc Prev. 2021 Mar;28(2):129-139. doi: 10.1007/s40292-021-00439-9. Epub 2021 Feb 26. High Blood Press Cardiovasc Prev. 2021. PMID: 33635533 Free PMC article. Review.
-
The concern about ACE/ARB and COVID-19: Time to hold your horses!J Am Pharm Assoc (2003). 2020 Nov-Dec;60(6):e88-e90. doi: 10.1016/j.japh.2020.06.026. Epub 2020 Jul 31. J Am Pharm Assoc (2003). 2020. PMID: 32747165 Free PMC article.
-
Angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) may be safe for COVID-19 patients.BMC Infect Dis. 2021 Jan 25;21(1):114. doi: 10.1186/s12879-021-05821-5. BMC Infect Dis. 2021. PMID: 33494713 Free PMC article.
-
Angiotensin-converting enzyme 2 and kidney diseases in the era of coronavirus disease 2019.Korean J Intern Med. 2021 Mar;36(2):247-262. doi: 10.3904/kjim.2020.355. Epub 2020 Oct 16. Korean J Intern Med. 2021. PMID: 33617712 Free PMC article. Review.
Cited by
-
Receptor Interactions of Angiotensin II and Angiotensin Receptor Blockers-Relevance to COVID-19.Biomolecules. 2021 Jul 3;11(7):979. doi: 10.3390/biom11070979. Biomolecules. 2021. PMID: 34356603 Free PMC article.
-
Marine Alga Ulva fasciata-Derived Molecules for the Potential Treatment of SARS-CoV-2: An In Silico Approach.Mar Drugs. 2022 Sep 19;20(9):586. doi: 10.3390/md20090586. Mar Drugs. 2022. PMID: 36135775 Free PMC article.
-
New Advances in Short Peptides: Looking Forward.Molecules. 2022 Jun 6;27(11):3635. doi: 10.3390/molecules27113635. Molecules. 2022. PMID: 35684571 Free PMC article.
-
Actions of Novel Angiotensin Receptor Blocking Drugs, Bisartans, Relevant for COVID-19 Therapy: Biased Agonism at Angiotensin Receptors and the Beneficial Effects of Neprilysin in the Renin Angiotensin System.Molecules. 2022 Jul 29;27(15):4854. doi: 10.3390/molecules27154854. Molecules. 2022. PMID: 35956801 Free PMC article.
-
SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention.Microorganisms. 2021 Mar 4;9(3):525. doi: 10.3390/microorganisms9030525. Microorganisms. 2021. PMID: 33806624 Free PMC article. Review.
References
-
- Timmermans P.B., Duncia J.V., Carini D.J., Chiu A.Τ., Wong P.C., Wexler R.R., Smith R.D. Discovery of Losartan, the first angiotensin II receptor antagonist. J. Hum. Hypertens. 1995;9:3–18. - PubMed
-
- Khairnar A.K., Baviskar D.T., Jain D.K. Angiotensin II Receptor Blockers: An Overview. Int. J. Pharm. Sci. 2012;4:50–56.
-
- Moore G.J., Smith J., Baylis B., Matsoukas J. Design and pharmacology of peptide mimetics. Adv. Pharmacol. 1995;33:91–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

