The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules
- PMID: 33504104
- PMCID: PMC7865374
- DOI: 10.3390/molecules26030627
The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules
Abstract
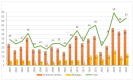
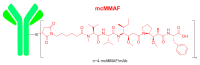
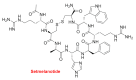
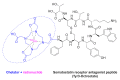
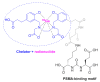
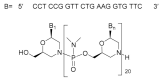
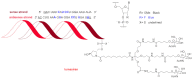
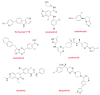
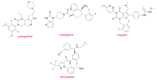
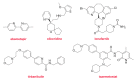
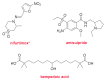
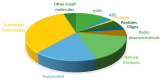
Although the pharmaceutical industry will remember 2020 as the year of COVID-19, it is important to highlight that this year has been the second-best-together with 1996-in terms of the number of drugs accepted by the US Food and Drug Administration (FDA). Each of these two years witnessed the authorization of 53 drugs-a number surpassed only in 2018 with 59 pharmaceutical agents. The 53 approvals in 2020 are divided between 40 new chemical entities and 13 biologic drugs (biologics). Of note, ten monoclonal antibodies, two antibody-drug conjugates, three peptides, and two oligonucleotides have been approved in 2020. Close inspection of the so-called small molecules reveals the significant presence of fluorine atoms and/or nitrogen aromatic heterocycles. This report analyzes the 53 new drugs of the 2020 harvest from a strictly chemical perspective, as it did for those authorized in the previous four years. On the basis of chemical structure alone, the drugs that received approval in 2020 are classified as the following: biologics (antibodies, antibody-drug conjugates, and proteins); TIDES (peptide and oligonucleotides); natural products; fluorine-containing molecules; nitrogen aromatic heterocycles; and other small molecules.
Keywords: API; CBER; CDER; COVID-19; TIDES; antibodies; antibody–drug conjugate; biologics; chemical entities; drug discovery; fluorine-based drugs; natural products; nitrogen aromatic heterocycles; oligonucleotides; peptides; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- U.S. Food and Drug Administration (FDA) [(accessed on 31 December 2020)]; Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and....
-
- Jarvis L.M. The new drugs of 2019. [(accessed on 20 January 2020)];Chem. Eng. News. 2020 98:30–36. Available online: https://cen.acs.org/pharmaceuticals/drug-development/new-drugs-2019/98/i3.
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2021)]; Available online: https://www.fda.gov/vaccines-blood-biologics/development-approval-proces....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

