Immunogenicity and safety of rapid scheme vaccination against tick-borne encephalitis in HIV-1 infected persons
- PMID: 33504405
- PMCID: PMC8060836
- DOI: 10.1017/S0950268821000194
Immunogenicity and safety of rapid scheme vaccination against tick-borne encephalitis in HIV-1 infected persons
Abstract
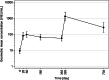
Tick-borne encephalitis (TBE) is a vector-borne infection associated with a variety of potentially serious complications and sequelae. Vaccination against TBE is strongly recommended for people living in endemic areas. There are two TBE vaccination schemes - standard and rapid - which differ in the onset of protection. With vaccination in a rapid schedule, protection starts as early as 4 weeks after the first dose and is therefore especially recommended for non-immune individuals travelling to endemic areas. Both schemes work reliably in immunocompetent individuals, but only little is known about how TBE vaccination works in people with HIV infection. Our aim was to assess the immunogenicity and safety of the rapid scheme of TBE vaccination in HIV-1 infected individuals. Concentrations of TBE-specific IgG > 126 VIEU/ml were considered protective. The seroprotection rate was 35.7% on day 28 and 39.3% on day 60. There were no differences between responders and non-responders in baseline and nadir CD4 + T lymphocytes. No serious adverse events were observed after vaccination. The immunogenicity of the TBE vaccination was unsatisfactory in our study and early protection was only achieved in a small proportion of vaccinees. Therefore, TBE vaccination with the rapid scheme cannot be recommended for HIV-1 infected individuals.
Keywords: Antibodies; HIV; rapid scheme; tick-borne encephalitis; vaccination.
Conflict of interest statement
None.
Figures
Similar articles
-
Magnitude and Functional Profile of the Human CD4+ T Cell Response throughout Primary Immunization with Tick-Borne Encephalitis Virus Vaccine.J Immunol. 2020 Feb 15;204(4):914-922. doi: 10.4049/jimmunol.1901115. Epub 2020 Jan 10. J Immunol. 2020. PMID: 31924650 Clinical Trial.
-
Long-term immunity after vaccination against tick-borne encephalitis with Encepur using the rapid vaccination schedule.Int J Med Microbiol. 2004 Apr;293 Suppl 37:130-3. doi: 10.1016/s1433-1128(04)80023-8. Int J Med Microbiol. 2004. PMID: 15146994 Clinical Trial.
-
Immunological response in HIV-positive patients vaccinated against tick-borne encephalitis.Infection. 2003 Jan;31(1):45-6. doi: 10.1007/s15010-002-2020-6. Infection. 2003. PMID: 12590332
-
Immunogenicity and safety of the tick-borne encephalitis vaccination (2009-2019): A systematic review.Travel Med Infect Dis. 2020 Sep-Oct;37:101876. doi: 10.1016/j.tmaid.2020.101876. Epub 2020 Sep 12. Travel Med Infect Dis. 2020. PMID: 32931931
-
Tick-borne encephalitis in China: A review of epidemiology and vaccines.Vaccine. 2017 Mar 1;35(9):1227-1237. doi: 10.1016/j.vaccine.2017.01.015. Epub 2017 Jan 30. Vaccine. 2017. PMID: 28153343 Review.
Cited by
-
A combined cross-sectional analysis and case-control study evaluating tick-borne encephalitis vaccination coverage, disease and vaccine effectiveness in children and adolescents, Switzerland, 2005 to 2022.Euro Surveill. 2024 May;29(18):2300558. doi: 10.2807/1560-7917.ES.2024.29.18.2300558. Euro Surveill. 2024. PMID: 38699900 Free PMC article.
-
Retrospective, matched case-control analysis of tickborne encephalitis vaccine effectiveness by booster interval, Switzerland 2006-2020.BMJ Open. 2022 Apr 22;12(4):e061228. doi: 10.1136/bmjopen-2022-061228. BMJ Open. 2022. PMID: 35459683 Free PMC article.
References
-
- Taba P et al. (2017) EAN Consensus review on prevention, diagnosis and management of tick-borne encephalitis. European Journal of Neurology 24, 1214–e1261. - PubMed
-
- Bogovic P et al. (2018) Factors associated with severity of tick-borne encephalitis: a prospective observational study. Travel Medicine and Infectious Disease 26, 25–31. - PubMed
-
- Heinz FX et al. (2007) Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25, 7559–7567. - PubMed
-
- Steffen R (2016) Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. Journal of Travel Medicine 23, 1–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials