Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: a Systematic Review and Meta-analysis
- PMID: 33504593
- PMCID: PMC8091856
- DOI: 10.1128/JCM.02881-20
Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: a Systematic Review and Meta-analysis
Abstract
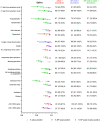
Nasopharyngeal (NP) swabs are considered the highest-yield sample for diagnostic testing for respiratory viruses, including SARS-CoV-2. The need to increase capacity for SARS-CoV-2 testing in a variety of settings, combined with shortages of sample collection supplies, have motivated a search for alternative sample types with high sensitivity. We systematically reviewed the literature to understand the performance of alternative sample types compared to NP swabs. We systematically searched PubMed, Google Scholar, medRxiv, and bioRxiv (last retrieval 1 October 2020) for comparative studies of alternative specimen types (saliva, oropharyngeal [OP], and nasal [NS] swabs) versus NP swabs for SARS-CoV-2 diagnosis using nucleic acid amplification testing (NAAT). A logistic-normal random-effects meta-analysis was performed to calculate % positive alternative-specimen, % positive NP, and % dual positives overall and in subgroups. The QUADAS 2 tool was used to assess bias. From 1,253 unique citations, we identified 25 saliva, 11 NS, 6 OP, and 4 OP/NS studies meeting inclusion criteria. Three specimen types captured lower % positives (NS [82%, 95% CI: 73 to 90%], OP [84%, 95% CI: 57 to 100%], and saliva [88%, 95% CI: 81 to 93%]) than NP swabs, while combined OP/NS matched NP performance (97%, 95% CI: 90 to 100%). Absence of RNA extraction (saliva) and utilization of a more sensitive NAAT (NS) substantially decreased alternative-specimen yield of positive samples. NP swabs remain the gold standard for diagnosis of SARS-CoV-2, although alternative specimens are promising. Much remains unknown about the impact of variations in specimen collection, processing protocols, and population (pediatric versus adult, late versus early in disease course), such that head-to head studies of sampling strategies are urgently needed.
Keywords: COVID-19; molecular diagnostic; nasal swab; nasopharyngeal swab; oropharyngeal swab; saliva.
Copyright © 2021 American Society for Microbiology.
Figures





References
-
- U.S. Food and Drug Administration, Center for Devices and Radiological Health. 2020. FAQs on testing for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-dev.... Accessed 12 October 2020.
-
- Centers for Disease Control and Prevention. 2020. Information for laboratories about coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed 4 October 2020.
-
- Wong SCY, Tse H, Siu HK, Kwong TS, Chu MY, Yau FYS, Cheung IYY, Tse CWS, Poon KC, Cheung KC, Wu TC, Chan JWM, Cheuk W, Lung DC. 2020. Posterior oropharyngeal saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71:2939–2946. 10.1093/cid/ciaa797. - DOI - PMC - PubMed
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

