Blockade of PD-1/PD-L1 Pathway Enhances the Antigen-Presenting Capacity of Fibrocytes
- PMID: 33504617
- PMCID: PMC7939041
- DOI: 10.4049/jimmunol.2000909
Blockade of PD-1/PD-L1 Pathway Enhances the Antigen-Presenting Capacity of Fibrocytes
Abstract
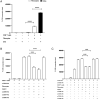
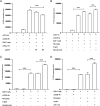
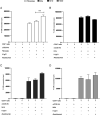
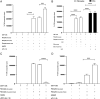
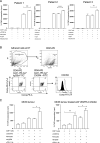
Fibrocytes, a distinct population of collagen-producing, monocyte-derived cells, are involved in wound healing as well as fibrotic diseases. Recently, fibrocytes have been revealed to play a role in the tumor microenvironment, particularly under antiangiogenic therapy. In addition, combination cancer immunotherapy with immune checkpoint inhibitor and antiangiogenic agents have been developed for various cancers in the clinical setting, although the immunological background is not clear. In the current study, we aimed to determine the function of fibrocytes in tumor immunity induced by immune checkpoint inhibitor therapy. Human and murine fibrocytes were generated from PBMCs and lungs, respectively. The expression of costimulatory and inhibitory molecules on fibrocytes was examined by flow cytometry. The stimulation of CD8+ T cells by fibrocytes was examined in MLRs with a 3H-thymidine incorporation assay. Fibrocytes expressed CD80low and CD86high as a costimulatory molecule, and expressed PD-L1high, but not PD-L2, as a coinhibitory molecule. Without any stimulation, fibrocytes strongly enhanced the proliferation of CD8+ T cells in mice and humans. Treatment with anti-CD86 and -CD54 Abs inhibited the growth of CD8+ T cells induced by fibrocytes. Anti-PD-L1 Ab further enhanced the proliferation of CD8+ T cells, even in the OVA-specific MLR with OT-1Rag-/- mice. Importantly, fibrocytes derived from PBMCs of patients with lung adenocarcinoma or murine MC38 tumors augmented the proliferation of CD8+ T cells with PD-L1 blockade. These results suggest that fibrocytes infiltrating tumor sites may play a role in the antitumor immunity mediated by CD8+ T cells when the activity is further enhanced by PD-L1/PD-1 blockade.
Copyright © 2021 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

References
-
- Bellini, A., Mattoli S.. 2007. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab. Invest. 87: 858–870. - PubMed
-
- Aono, Y., Kishi M., Yokota Y., Azuma M., Kinoshita K., Takezaki A., Sato S., Kawano H., Kishi J., Goto H., et al. . 2014. Role of platelet-derived growth factor/platelet-derived growth factor receptor axis in the trafficking of circulating fibrocytes in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 51: 793–801. - PubMed
-
- Gomperts, B. N., Strieter R. M.. 2007. Fibrocytes in lung disease. J. Leukoc. Biol. 82: 449–456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

