Reduction of alternative electron acceptors drives biofilm formation in Shewanella algae
- PMID: 33504806
- PMCID: PMC7840931
- DOI: 10.1038/s41522-020-00177-1
Reduction of alternative electron acceptors drives biofilm formation in Shewanella algae
Abstract
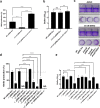
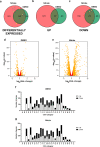
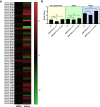
Shewanella spp. possess a broad respiratory versatility, which contributes to the occupation of hypoxic and anoxic environmental or host-associated niches. Here, we observe a strain-specific induction of biofilm formation in response to supplementation with the anaerobic electron acceptors dimethyl sulfoxide (DMSO) and nitrate in a panel of Shewanella algae isolates. The respiration-driven biofilm response is not observed in DMSO and nitrate reductase deletion mutants of the type strain S. algae CECT 5071, and can be restored upon complementation with the corresponding reductase operon(s) but not by an operon containing a catalytically inactive nitrate reductase. The distinct transcriptional changes, proportional to the effect of these compounds on biofilm formation, include cyclic di-GMP (c-di-GMP) turnover genes. In support, ectopic expression of the c-di-GMP phosphodiesterase YhjH of Salmonella Typhimurium but not its catalytically inactive variant decreased biofilm formation. The respiration-dependent biofilm response of S. algae may permit differential colonization of environmental or host niches.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparative Genomics of Cyclic di-GMP Metabolism and Chemosensory Pathways in Shewanella algae Strains: Novel Bacterial Sensory Domains and Functional Insights into Lifestyle Regulation.mSystems. 2022 Apr 26;7(2):e0151821. doi: 10.1128/msystems.01518-21. Epub 2022 Mar 21. mSystems. 2022. PMID: 35311563 Free PMC article.
-
Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi.Biochemistry. 2012 Mar 13;51(10):2087-99. doi: 10.1021/bi201753f. Epub 2012 Mar 5. Biochemistry. 2012. PMID: 22360279
-
PdeB, a cyclic Di-GMP-specific phosphodiesterase that regulates Shewanella oneidensis MR-1 motility and biofilm formation.J Bacteriol. 2013 Sep;195(17):3827-33. doi: 10.1128/JB.00498-13. Epub 2013 Jun 21. J Bacteriol. 2013. PMID: 23794617 Free PMC article.
-
Shewanella biofilm development and engineering for environmental and bioenergy applications.Curr Opin Chem Biol. 2020 Dec;59:84-92. doi: 10.1016/j.cbpa.2020.05.004. Epub 2020 Aug 1. Curr Opin Chem Biol. 2020. PMID: 32750675 Review.
-
Quorum sensing and biofilm formation in mycobacteria: role of c-di-GMP and methods to study this second messenger.IUBMB Life. 2014 Dec;66(12):823-34. doi: 10.1002/iub.1339. Epub 2014 Dec 26. IUBMB Life. 2014. PMID: 25546058 Review.
Cited by
-
Investigation and pathogenetic testing of Shewanella spp. positive diarrhea cases in Beijing, China.Sci Rep. 2025 Aug 19;15(1):30334. doi: 10.1038/s41598-025-15865-1. Sci Rep. 2025. PMID: 40830642 Free PMC article.
-
Is biofilm formation intrinsic to the origin of life?Environ Microbiol. 2023 Jan;25(1):26-39. doi: 10.1111/1462-2920.16179. Epub 2022 Sep 7. Environ Microbiol. 2023. PMID: 36655713 Free PMC article.
-
A recently isolated human commensal Escherichia coli ST10 clone member mediates enhanced thermotolerance and tetrathionate respiration on a P1 phage-derived IncY plasmid.Mol Microbiol. 2021 Feb;115(2):255-271. doi: 10.1111/mmi.14614. Epub 2020 Oct 12. Mol Microbiol. 2021. PMID: 32985020 Free PMC article.
-
Biochemical characterization and mercury methylation capacity of Geobacter sulfurreducens biofilms grown in media containing iron hydroxide or fumarate.Biofilm. 2023 Jul 29;6:100144. doi: 10.1016/j.bioflm.2023.100144. eCollection 2023 Dec 15. Biofilm. 2023. PMID: 37583615 Free PMC article.
-
Out of control: The need for standardised solvent approaches and data reporting in antibiofilm assays incorporating dimethyl-sulfoxide (DMSO).Biofilm. 2022 Aug 17;4:100081. doi: 10.1016/j.bioflm.2022.100081. eCollection 2022 Dec. Biofilm. 2022. PMID: 36060119 Free PMC article.
References
-
- Nealson, K. H. & Scott, J. Ecophysiology of the genus Shewanella. In The Prokaryotes. 1133–1151 (Springer New York, 2006).
-
- Martín-Rodríguez AJ, Suárez-Mesa A, Artiles-Campelo F, Römling U, Hernández M. Multilocus sequence typing of Shewanella algae isolates identifies disease-causing Shewanella chilikensis strain 6I4. FEMS Microbiol. Ecol. 2019;95:fiy210. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

