The inflammatory response induced by Pseudomonas aeruginosa in macrophages enhances apoptotic cell removal
- PMID: 33504816
- PMCID: PMC7841155
- DOI: 10.1038/s41598-021-81557-1
The inflammatory response induced by Pseudomonas aeruginosa in macrophages enhances apoptotic cell removal
Abstract
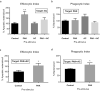
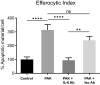
Pathogens phagocytosis and the uptake of apoptotic cells (efferocytosis) are essential macrophages tasks, classically considered as mutually exclusive. Macrophages have been observed to polarize into either pro-inflammatory/microbicidal or anti-inflammatory/efferocytic phenotypes. However, macrophage functions have shown to be more complex. Furthermore, little is known about the regulation of efferocytosis under inflammatory conditions. In this study, we elucidate the modulation of the macrophage efferocytic function during an inflammatory stimulus. We find that bone marrow-derived macrophages (BMDM) are very efficient in engulfing both the bacterial pathogen Pseudomonas aeruginosa and apoptotic cells. BMDM showed a high bactericidal capacity unaffected by the concomitant presence of apoptotic material. Plasticity in macrophage programming, in response to changing environmental cues, may modulate efferocytic capability. In this work, we further show that, after phagocyting and processing Pseudomonas aeruginosa, macrophages highly increase their efferocytic capacity without affecting their phagocytic function. Moreover, we demonstrate that Pseudomonas aeruginosa enhances efferocytosis of these phagocytes through the IL-6 signaling pathway. Our results show that the inflammatory response generated by the bacterial processing enhances these macrophages' capacity to control inflammation through an increased efferocytosis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

