Cyclophosphamide arrhythmogenicitytesting using human-induced pluripotent stem cell-derived cardiomyocytes
- PMID: 33504826
- PMCID: PMC7841168
- DOI: 10.1038/s41598-020-79085-5
Cyclophosphamide arrhythmogenicitytesting using human-induced pluripotent stem cell-derived cardiomyocytes
Abstract
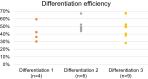
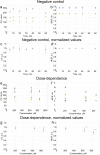
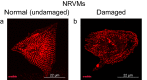
Cyclophosphamide (CP) is an anticancer drug, an alkylating agent. Cardiotoxicity of CP is associated with one of its metabolites, acrolein, and clinical cardiotoxicity manifestations are described for cases of taking CP in high doses. Nevertheless, modern arrhythmogenicity prediction assays in vitro include evaluation of beat rhythm and rate as well as suppression of cardiac late markers after acute exposure to CP, but not its metabolites. The mechanism of CP side effects when taken at low doses (i.e., < 100 mg/kg), especially at the cellular level, remains unclear. In this study conduction properties and cytoskeleton structure of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) obtained from a healthy donor under CP were evaluated. Arrhythmogenicity testing including characterization of 3 values: conduction velocity, maximum capture rate (MCR) measurements and number of occasions of re-entry on a standard linear obstacle was conducted and revealed MCR decrease of 25% ± 7% under CP. Also, conductivity area reduced by 34 ± 15%. No effect of CP on voltage-gated ion channels was found. Conduction changes (MCR and conductivity area decrease) are caused by exposure time-dependent alpha-actinin disruption detected both in hiPSC-CMs and neonatal ventricular cardiomyocytes in vitro. Deviation from the external stimulus frequency and appearance of non-conductive areas in cardiac tissue under CP is potentially arrhythmogenic and could develop arrhythmic effects in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Iqubal A, Haque SE, Sharma S, Ansari MA, Khan V, Iqubal MK. Clinical updates on drug—induced cardiotoxicity. Int. J. Pharm. Sci. Res. 2018 doi: 10.13040/IJPSR.0975-8232.9(1).16-26. - DOI
-
- Steingo, L. Cardiovascular complications of chemotherapy: A synopsis. SA Hear.9, (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

