COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation
- PMID: 33505220
- PMCID: PMC7811571
- DOI: 10.1155/2021/8874339
COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation
Abstract
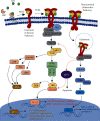
Causes of mortality from COVID-19 include respiratory failure, heart failure, and sepsis/multiorgan failure. TLR4 is an innate immune receptor on the cell surface that recognizes pathogen-associated molecular patterns (PAMPs) including viral proteins and triggers the production of type I interferons and proinflammatory cytokines to combat infection. It is expressed on both immune cells and tissue-resident cells. ACE2, the reported entry receptor for SARS-CoV-2, is only present on ~1-2% of the cells in the lungs or has a low pulmonary expression, and recently, the spike protein has been proposed to have the strongest protein-protein interaction with TLR4. Here, we review and connect evidence for SARS-CoV-1 and SARS-CoV-2 having direct and indirect binding to TLR4, together with other viral precedents, which when combined shed light on the COVID-19 pathophysiological puzzle. We propose a model in which the SARS-CoV-2 spike glycoprotein binds TLR4 and activates TLR4 signalling to increase cell surface expression of ACE2 facilitating entry. SARS-CoV-2 also destroys the type II alveolar cells that secrete pulmonary surfactants, which normally decrease the air/tissue surface tension and block TLR4 in the lungs thus promoting ARDS and inflammation. Furthermore, SARS-CoV-2-induced myocarditis and multiple-organ injury may be due to TLR4 activation, aberrant TLR4 signalling, and hyperinflammation in COVID-19 patients. Therefore, TLR4 contributes significantly to the pathogenesis of SARS-CoV-2, and its overactivation causes a prolonged or excessive innate immune response. TLR4 appears to be a promising therapeutic target in COVID-19, and since TLR4 antagonists have been previously trialled in sepsis and in other antiviral contexts, we propose the clinical trial testing of TLR4 antagonists in the treatment of severe COVID-19. Also, ongoing clinical trials of pulmonary surfactants in COVID-19 hold promise since they also block TLR4.
Copyright © 2021 Mohamed M. Aboudounya and Richard J. Heads.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- World health organization. Coronavirus disease (COVID-2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

