Effects of Melatonin on Anterior Pituitary Plasticity: A Comparison Between Mammals and Teleosts
- PMID: 33505357
- PMCID: PMC7831660
- DOI: 10.3389/fendo.2020.605111
Effects of Melatonin on Anterior Pituitary Plasticity: A Comparison Between Mammals and Teleosts
Abstract
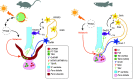
Melatonin is a key hormone involved in the photoperiodic signaling pathway. In both teleosts and mammals, melatonin produced in the pineal gland at night is released into the blood and cerebrospinal fluid, providing rhythmic information to the whole organism. Melatonin acts via specific receptors, allowing the synchronization of daily and annual physiological rhythms to environmental conditions. The pituitary gland, which produces several hormones involved in a variety of physiological processes such as growth, metabolism, stress and reproduction, is an important target of melatonin. Melatonin modulates pituitary cellular activities, adjusting the synthesis and release of the different pituitary hormones to the functional demands, which changes during the day, seasons and life stages. It is, however, not always clear whether melatonin acts directly or indirectly on the pituitary. Indeed, melatonin also acts both upstream, on brain centers that control the pituitary hormone production and release, as well as downstream, on the tissues targeted by the pituitary hormones, which provide positive and negative feedback to the pituitary gland. In this review, we describe the known pathways through which melatonin modulates anterior pituitary hormonal production, distinguishing indirect effects mediated by brain centers from direct effects on the anterior pituitary. We also highlight similarities and differences between teleosts and mammals, drawing attention to knowledge gaps, and suggesting aims for future research.
Keywords: adenohypophysis; endocrinology; light; melatonin; melatonin receptors; photoperiod; plasticity; seasonal reproduction.
Copyright © 2021 Ciani, Haug, Maugars, Weltzien, Falcón and Fontaine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Falcón J, Zohar Y. Photoperiodism in fish. In: Encyclopedia of Reproduction. New York (USA): Elsevier; (2018). p. 400–8. 10.1016/B978-0-12-809633-8.20584-0 - DOI
-
- Reiter RJ, Tan D-X, Sharma R. Historical Perspective and Evaluation of the Mechanisms by which Melatonin Mediates Seasonal Reproduction in Mammals. Melatonin Res (2018) 1(1):59–77. 10.32794/mr11250004 - DOI
-
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, et al. The Melatonin Rhythm-generating Enzyme: Molecular Regulation of Serotonin N-acetyltransferase in the Pineal Gland. Recent Prog Horm Res (1997) 52:307–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

