Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions
- PMID: 33505393
- PMCID: PMC7829348
- DOI: 10.3389/fimmu.2020.594150
Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions
Abstract
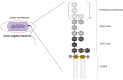
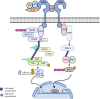
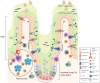
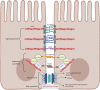
Diet-induced metabolic endotoxemia is an important factor in the development of many chronic diseases in animals and man. The gut epithelium is an efficient barrier that prevents the absorption of liposaccharide (LPS). Structural changes to the intestinal epithelium in response to dietary alterations allow LPS to enter the bloodstream, resulting in an increase in the plasma levels of LPS (termed metabolic endotoxemia). LPS activates Toll-like receptor-4 (TLR4) leading to the production of numerous pro-inflammatory cytokines and, hence, low-grade systemic inflammation. Thus, metabolic endotoxemia can lead to several chronic inflammatory conditions. Obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD) can also cause an increase in gut permeability and potential pharmacological and dietary interventions could be used to reduce the chronic low-grade inflammation associated with endotoxemia.
Keywords: Toll-like receptor; antimicrobial peptides; gut permeability; high-fat diet; lipopolysaccharide; metabolic endotoxemia.
Copyright © 2021 Mohammad and Thiemermann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
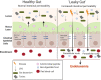
References
-
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic Endotoxemia Initiates Obesity and InsulinResistance. Diabetes (2007) 56(7):1761–72. - PubMed
-
- Schultz C. Lipopolysaccharide, structure and biological effects. Gen Intern Med Clin Innov (2018) 3:1–2. 10.15761/GIMCI.1000152 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

