In Vitro Tests for Assessing the Neutralizing Ability of Snake Antivenoms: Toward the 3Rs Principles
- PMID: 33505403
- PMCID: PMC7829219
- DOI: 10.3389/fimmu.2020.617429
In Vitro Tests for Assessing the Neutralizing Ability of Snake Antivenoms: Toward the 3Rs Principles
Abstract
There is an urgent need to strengthen the implementation of the 3Rs principle (Replacement, Reduction and Refinement) in the use of experimental animals in toxinological research and in the assessment of the neutralizing efficacy of snake antivenoms. This is a challenging task owing to the inherent complexity of snake venoms. The state of the art on this topic is hereby reviewed, with emphasis on the studies in which a correlation has been observed between in vivo toxicity tests and in vitro surrogate assays, particularly in the study of lethal activity of venoms and its neutralization. Correlations have been described with some venoms-antivenoms when using: (a) enzyme immunoassays, (b) hemagglutination, (c) enzyme assays (proteinase, phospholipase A2), (d) in vitro coagulant effect on plasma, (e) cell culture assays for cytotoxicity, (f) functional assays for assessing neurotoxicity in vitro, (g) use of hens' eggs, and (h) antivenomics. Additionally, the routine introduction of analgesia in these assays and the design of more 'humane' protocols for the lethality test are being pursued. It is expected that the next years will witness a growing awareness of the relevance of the 3Rs principles in antivenom testing, and that new in vitro alternatives and more 'humane' experimental designs will emerge in this field.
Keywords: 3Rs; analgesia; antivenoms; in vitro assays; lethality assays; neutralization; snake venoms.
Copyright © 2021 Gutiérrez, Vargas, Segura, Herrera, Villalta, Solano, Sánchez, Herrera and León.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

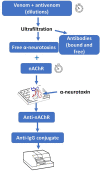

References
-
- Gutiérrez JM. Snakebite Envenomation as a Neglected Tropical Disease: New Impetus For Confronting an Old Scourge. In: Mackessy SP, editor. Snake Venoms and Toxins, 2nd Edition Boca Raton, Florida, USA: CRC Press (2020).
-
- World Health Organization Snakebite Envenoming. Strategy for Prevention and Control. Geneva: World Health Organization; (2019). - PubMed
-
- World Health Organization WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Geneva: World Health Organization; (2018). Available at: http://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

