Characterisation of Cellulose Synthase Like F6 (CslF6) Mutants Shows Altered Carbon Metabolism in β-D-(1,3;1,4)-Glucan Deficient Grain in Brachypodium distachyon
- PMID: 33505412
- PMCID: PMC7829222
- DOI: 10.3389/fpls.2020.602850
Characterisation of Cellulose Synthase Like F6 (CslF6) Mutants Shows Altered Carbon Metabolism in β-D-(1,3;1,4)-Glucan Deficient Grain in Brachypodium distachyon
Abstract
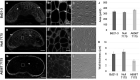
Brachypodium distachyon is a small, fast growing grass species in the Pooideae subfamily that has become established as a model for other temperate cereals of agricultural significance, such as barley (Hordeum vulgare) and wheat (Triticum aestivum). The unusually high content in whole grains of β-D-(1,3;1,4)-glucan or mixed linkage glucan (MLG), considered a valuable dietary fibre due to its increased solubility in water compared with cellulose, makes B. distachyon an attractive model for these polysaccharides. The carbohydrate composition of grain in B. distachyon is interesting not only in understanding the synthesis of MLG, but more broadly in the mechanism(s) of carbon partitioning in cereal grains. Several mutants in the major MLG synthase, cellulose synthase like (CSL) F6, were identified in a screen of a TILLING population that show a loss of function in vitro. Surprisingly, loss of cslf6 synthase capacity appears to have a severe impact on survival, growth, and development in B. distachyon in contrast to equivalent mutants in barley and rice. One mutant, A656T, which showed milder growth impacts in heterozygotes shows a 21% (w/w) reduction in average grain MLG and more than doubling of starch compared with wildtype. The endosperm architecture of grains with the A656T mutation is altered, with a reduction in wall thickness and increased deposition of starch in larger granules than typical of wildtype B. distachyon. Together these changes demonstrate an alteration in the carbon storage of cslf6 mutant grains in response to reduced MLG synthase capacity and a possible cross-regulation with starch synthesis which should be a focus in future work in composition of these grains. The consequences of these findings for the use of B. distachyon as a model species for understanding MLG synthesis, and more broadly the implications for improving the nutritional value of cereal grains through alteration of soluble dietary fibre content are discussed.
Keywords: 3;1; 4)-glucan; Brachypodium distachyon; cell wall; endosperm; starch; β-D-(1.
Copyright © 2021 Bain, van de Meene, Costa and Doblin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources

