Astaxanthin Alleviates Ochratoxin A-Induced Cecum Injury and Inflammation in Mice by Regulating the Diversity of Cecal Microbiota and TLR4/MyD88/NF- κ B Signaling Pathway
- PMID: 33505592
- PMCID: PMC7806395
- DOI: 10.1155/2021/8894491
Astaxanthin Alleviates Ochratoxin A-Induced Cecum Injury and Inflammation in Mice by Regulating the Diversity of Cecal Microbiota and TLR4/MyD88/NF- κ B Signaling Pathway
Abstract
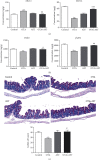
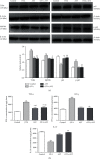
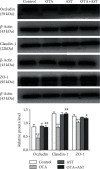
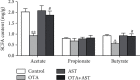
Ochratoxin A (OTA) is a common environmental pollutant found in a variety of foods and grains, and excessive OTA consumption causes serious global health effects on animals and humans. Astaxanthin (AST) is a natural carotenoid that has anti-inflammatory, antiapoptotic, immunomodulatory, antitumor, antidiabetes, and other biological activities. The present study is aimed at investigating the effects of AST on OTA-induced cecum injury and its mechanism of action. Eighty C57 mice were randomly divided into four groups, including the control group, OTA group (5 mg/kg body weight), AST group (100 mg/kg body weight), and AST intervention group (100 mg/kg body weight AST+5 mg/kg body weight OTA). It was found that AST decreased the endotoxin content, effectively prevented the shortening of mouse cecum villi, and increased the expression levels of tight junction (TJ) proteins, consisting of occludin, claudin-1, and zonula occludens-1 (ZO-1). AST increased the number of goblet cells, the contents of mucin-2 (MUC2), and defensins (Defa5 and β-pD2) significantly, while the expression of mucin-1 (MUC1) decreased significantly. The 16S rRNA sequencing showed that AST affected the richness and diversity of cecum flora, decreased the proportion of lactobacillus, and also decreased the contents of short-chain fatty acids (SCFAs) (acetate and butyrate). In addition, AST significantly decreased the expression of TLR4, MyD88, and p-p65, while increasing the expression of p65. Meanwhile, the expression of inflammatory factors including TNF-α and INF-γ decreased, while the expression of IL-10 increased. In conclusion, AST reduced OTA-induced cecum injury by regulating the cecum barrier function and TLR4/MyD88/NF-κB signaling pathway.
Copyright © 2021 Yueli Chen et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Astaxanthin Protects OTA-Induced Lung Injury in Mice through the Nrf2/NF-κB Pathway.Toxins (Basel). 2019 Sep 17;11(9):540. doi: 10.3390/toxins11090540. Toxins (Basel). 2019. PMID: 31533259 Free PMC article.
-
Ginsenoside Rg1 alleviates ochratoxin A-induced liver inflammation in ducklings: Involvement of intestinal microbiota modulation and the TLR4/NF-κB pathway inhibition.Ecotoxicol Environ Saf. 2025 May;296:118186. doi: 10.1016/j.ecoenv.2025.118186. Epub 2025 Apr 14. Ecotoxicol Environ Saf. 2025. PMID: 40222107
-
Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers.Food Chem Toxicol. 2020 Mar;137:111139. doi: 10.1016/j.fct.2020.111139. Epub 2020 Jan 23. Food Chem Toxicol. 2020. PMID: 31981684
-
Astaxanthin as a Modulator of Nrf2, NF-κB, and Their Crosstalk: Molecular Mechanisms and Possible Clinical Applications.Molecules. 2022 Jan 14;27(2):502. doi: 10.3390/molecules27020502. Molecules. 2022. PMID: 35056816 Free PMC article. Review.
-
Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases.Biomed Pharmacother. 2022 Jan;145:112179. doi: 10.1016/j.biopha.2021.112179. Epub 2021 Nov 1. Biomed Pharmacother. 2022. PMID: 34736076 Review.
Cited by
-
Effects of nano-selenium on cecum microbial community and metabolomics in chickens challenged with Ochratoxin A.Front Vet Sci. 2023 Sep 5;10:1228360. doi: 10.3389/fvets.2023.1228360. eCollection 2023. Front Vet Sci. 2023. PMID: 37732141 Free PMC article.
-
Combined treatment with Rg1 and adipose-derived stem cells alleviates DSS-induced colitis in a mouse model.Stem Cell Res Ther. 2022 Jun 21;13(1):272. doi: 10.1186/s13287-022-02940-x. Stem Cell Res Ther. 2022. PMID: 35729638 Free PMC article.
-
Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids.Mar Drugs. 2021 Sep 23;19(10):531. doi: 10.3390/md19100531. Mar Drugs. 2021. PMID: 34677429 Free PMC article. Review.
-
Crosstalk between Mycotoxins and Intestinal Microbiota and the Alleviation Approach via Microorganisms.Toxins (Basel). 2022 Dec 6;14(12):859. doi: 10.3390/toxins14120859. Toxins (Basel). 2022. PMID: 36548756 Free PMC article. Review.
-
Sources, dynamics in vivo, and application of astaxanthin and lutein in laying hens: A review.Anim Nutr. 2023 Mar 2;13:324-333. doi: 10.1016/j.aninu.2023.02.008. eCollection 2023 Jun. Anim Nutr. 2023. PMID: 37207112 Free PMC article. Review.
References
-
- González-Arias C. A., Crespo-Sempere A., Marín S., Sanchis V., Ramos A. J. Modulation of the xenobiotic transformation system and inflammatory response by ochratoxin A exposure using a co-culture system of Caco-2 and HepG2 cells. Food and Chemical Toxicology. 2015;86:245–252. doi: 10.1016/j.fct.2015.10.007. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

